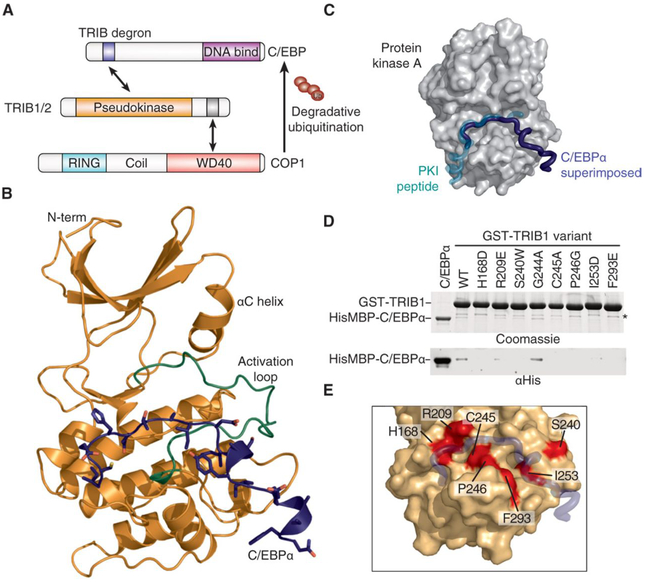
Fig. 1. The TRIB1-C/EBPα degron complex.
(A) Schematic illustrating degradation of C/EBPα by TRIB1-COP1. (B) Crystal structure of the TRIB1-C/EBPα degron complex, with TRIB1 shown predominantly in orange with a green activation loop and C/EBPα in blue. (C) Comparison of the C/EBPα binding mode (blue) with that of the prototypic substrate-like PKI (turquoise) in complex with PKA [gray surface; Protein Data Bank (PDB) 1ATP]. To generate the overlay, the TRIB1-C/EBPα complex structure was superimposed on the basis of the pseudokinase and kinase domains of TRIB1 and PKA, respectively. (D) Glutathione S-transferase (GST) pulldown of His6MBP-C/EBPα(53–75) by wild-type (WT) GST-TRIB1(84–372) and indicated mutants, separated by SDS–polyacrylamide gel electrophoresis (PAGE) and visualized by Coomassie blue staining (top) or anti-His6 immunoblotting (bottom). A nonspecific band that copurifies with GST-TRIB1 is indicated with an asterisk. (E) Structural representation of mutants that disrupt C/EBPα binding. TRIB1 is shown as an orange surface and C/EBPα in blue, with TRIB1 mutants that disrupt binding shown in red.