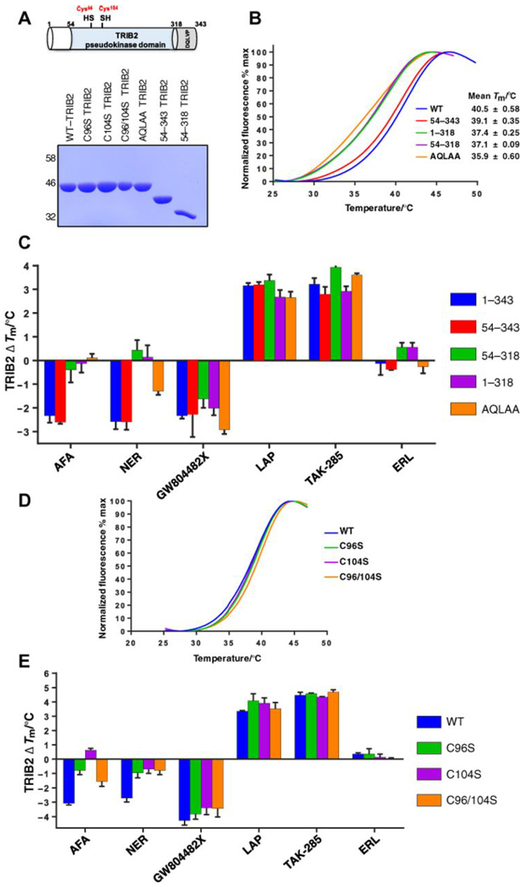
Fig. 2. TRIB2 thermal stability is modulated through Cys binding to covalent inhibitors.
(A) Top: Schematic cartoon of TRIB2 with domain boundaries numbered and cysteine residues highlighted (red). Bottom: SDS-PAGE of 5-μg recombinant TRIB2 proteins. (B) Thermal denaturation profiles of 5 μM WT-TRIB2 (amino acids 1 to 343), three truncated variants, and an AQLAA triple-point mutant. Representative curves for each protein and average Tm values (±SD) are shown, calculated from N = 3 experiments. (C) Thermal shift analysis of TRIB2 deletion and AQLAA proteins measured in the presence of a panel of compounds (20 μM). The change in Tm value (ΔTm) is reported from N = 3 experiments, each performed in triplicate. (D) Thermal denaturation profiles for purified TRIB2 and C96S, C104S, and C96/104S proteins. (E) Thermal shift analysis of TRIB2 Cys-mutated proteins measured in the presence of a panel of compounds (20 μM). The change in Tm value (ΔTm) is reported from N = 3 experiments.