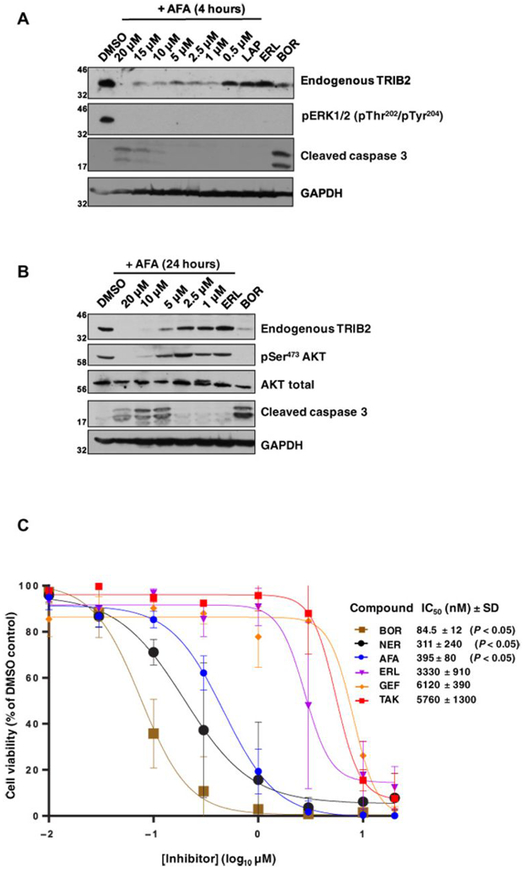
Fig. 5. Afatinib rapidly destabilizes endogenous TRIB2 and specifically induces caspase 3 cleavage and U937 cytotoxicity.
(A) Endogenous TRIB2 is destabilized in human U937 cells in a dose-dependent manner after a 4-hour exposure to afatinib. Cells were incubated with either 0.1% (v/v) DMSO or the indicated concentrations of afatinib, lapatinib (10 μM), erlotinib (10 μM), or the proteasome inhibitor bortezomib (10 μM) for 4 hours before lysis and immunoblotting of endogenous TRIB2, pERK, or cleaved caspase 3. GAPDH served as a loading control. (B) Endogenous TRIB2 is destabilized after exposure to afatinib for 24 hours, concomitant with reduced AKT phosphorylation at Ser473. After cell collection and lysis, whole-cell extracts were immunoblotted with the indicated antibodies. AKT and GAPDH served as loading controls; erlotinib and bortezomib were used at 10 μM. (C) The cytotoxicity of a panel of TRIB2-destabilizing small molecules (neratinib) was compared to EGFR inhibitors or the TRIB2 stabilizer TAK-285. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays were performed after 72 hours of compound exposure, with bortezomib used as a positive control. IC50 values (nM, mean ± SD) were derived from N = 3 independent experiments, each performed in triplicate. Statistical analysis confirmed a significant difference in cytotoxicity between afatinib and erlotinib (Student’s t test; P = 0.0104), between bortezomib and erlotinib (P = 0.0072), and between neratinib and erlotinib (P = 0.0104). Blots are representative of three independent experiments.