Abstract
The moss Physcomitrella patens receives increased scientific interest since its genome was sequenced a decade ago. As a bryophyte, it represents the first group of plants that evolved in a terrestrial habitat still without a vascular system that developed later in tracheophytes. It is easily transformable via homologous recombination, which enables the formation of targeted loss-of-function mutants. Even though genetics, development and life cycle in Physcomitrella are well studied nowadays, research on lipids in Physcomitrella is still underdeveloped. This review aims on presenting an overview on the state of the art of lipid research with a focus on membrane lipids, surface lipids and oxylipins. We discuss in this review that Physcomitrella possesses very interesting features regarding its membrane lipids. Here, the presence of very-long-chain polyunsaturated fatty acids (VLC-PUFA) still shows a closer similarity to marine microalgae than to vascular plants. Unlike algae, Physcomitrella has a cuticle comparable to vascular plants composed of cutin and waxes. The presence of VLC-PUFA in Physcomitrella also leads to a greater variability of signaling lipids even though the phytohormone jasmonic acid is not present in this organism, which is different to vascular plants. In summary, the research on lipids in Physcomitrella is still in its infancy, especially considering membrane lipids. We hope that this review will help to promote the further advancement of lipid research in this important model organism in the future, so we can better understand how lipids are involved in the evolution of land plants.
Keywords: Bryophytes, Membrane lipids, Oxylipins, Physcomitrella patens, Waxes
Introduction
Bryophytes, which include mosses, liverworts and hornworts, are a division of land plants with origins close to the earliest land-colonizing plants, which evolved about 450 million years ago (Clarke et�al. 2011). They represent the evolutionary link between marine plants, like algae, and later terrestrial vascular plants. Plant biology, however, focusses still primarily on vascular plants, especially seed plants. Bryophytes represent a different strategy for dealing with terrestrial conditions (Cuming 2012), which also reflects upon their molecular makeup, including lipids. Identifying the differences in lipid metabolism and function between bryophytes and vascular plants therefore can provide us with insights into these two types of plants developed differently over time on a molecular level. Furthermore, understanding the lipid composition of bryophytes could also give us insights into how marine organisms developed the capability to grow on land.
Of all bryophytes, the moss Physcomitrella patens is possibly the best studied species so far (Cove 2005). It was chosen as a model organism and its genome was sequenced in 2008 (Rensing et�al. 2008). However, by growing it at room temperature and long-day growth conditions (16 h light/8 h dark), as well as mechanically disrupting the moss tissue regularly, P. patens cultures can be maintained indefinitely without the need for spore production. The ‘Gransden’ strain of P. patens, the most commonly used strain in studies, has been grown continuously under laboratory conditions for over half a century now (Ashton and Cove 1977), which apparently resulted in a reduced general fertility compared to moss grown in nature (Schaefer and Zr�d 2001). Genetic modification is, compared to most other plant model organism, much easier when working with P. patens (Frank et�al. 2005). The organism possesses a highly effective DNA repair mechanism, allowing transformation of protoplasts via homologous recombination with an effectiveness similar to the yeast Saccharomyces cerevisiae (Schaefer et�al. 1991). This feature, combined with a haploid main growth stage, makes it much easier in P. patens to create targeted loss-of-function mutants than compared to vascular model plants like Arabidopsis thaliana. Interestingly, one of the first mutants that had been generated in P. patens was a loss-of-function mutant in a Δ6 desaturase affecting membrane lipid metabolism (Girke et�al. 1998).
Compared to higher plants the life cycle of the P. patens Gransden strain completes in 3–4 months and the moss grows in a rather simple developmental pattern (Engel 1968). The dominant phase of the moss is the haploid gametophyte. The cycle starts either via vegetative propagation from protoplasts and from disrupted tissue or via reproductive growth from spores (Cove et�al. 2006). The differentiation into the various developmental stages is phytohormone-dependent (Decker et�al. 2006). The germinating spore or the protoplast differentiates into the protonemal tissue (Fig.�1A). The protonema is the juvenile stage of the moss and grows in a network of filamentous cells composed of two different cell types: the assimilatory chloronemata (Fig.�1B) and the adventitious caulonemata (Fig.�1C) (Reski 1998). The chloronemal cells are the first cells that develop from the germinating spore. They contain many chloroplasts and are rather short. The chloronemal cells eventually give rise to the caulonemata. In contrast to the chloronemata the caulonemal cells are longer, thinner and contain fewer, less developed chloroplasts. Generation of side branches into secondary chloronemal and caulonemal cells leads to a interwoven filamentous protonemal network. The two described cell types can be identified by the characteristic orientation of the cross walls between two filaments to the cell axis. In chloronemal cells the cross wall orients perpendicular to the cell axis (Fig.�1B) while in caulonemal cells the cross wall shows an oblique orientation to the cell axis (Fig.�1C) (Reski 1998). The growth of the protonemal filaments happens by a serial division of the apical cells. Some of the side branches formed by the subapical cells develop into bud formation that eventually give rise to the adult structure of the moss. The emerging leafy shoot is the gametophore (Fig.�1D). The gametophore has a stem, leaf-like organs called phyllids and root-like organs called rhizoids (Reski 1998). Application of autumn-like conditions, meaning water overlay, short-day conditions (8 h light/16 h dark) and a temperature shift below 18�C, induces the formation of female and male gametangia, archegonia and antheridia, respectively, on top of the gametophore (Fig.�1E) (Engel 1968, Hohe et�al. 2002). Thanks to the moist environment, the motile male gametes (spermatozoids) can move from the male antheridia to the female archegonia. After fertilization, the spore capsule develops (Fig.�1F). This stage of the life cycle is the sporophyte, representing the only diploid stage in the moss development. After the spore capsule releases the haploid spores, the cycle starts over again.
Fig. 1.
Different developmental stages of P. patens. (A) A 3-week-old colony showing protonema growth that consists of chloronemal cells in the center and caulonemal cells in the periphery. (B) Chloronemal filaments with a characteristic cross wall oriented perpendicular to the cell axis (arrows). (C) Caulonemal filaments with a characteristic cross wall oriented oblique to the cell axis (arrows). (D) A leafy shoot, the gametophore, build-up of a stem, leaf-like organs, the phyllids and root-like organs, the rhizoids. (E) Close-up of the top of a gametophore, carrying the gametangia: the female archegonia (f) and the male antherida (m) (arrows). (F) Close-up of the top of a gametophore carrying the sporophyte with a mature spore capsule.
Even though P. patens has been established as a moss model organism for several years, many areas of research remain understudied compared to other model plants. Lipids in P. patens have so far been analyzed only scarcely, with a focus on primary lipid compounds like fatty acids (FA) or some lipid-derived signaling compounds. Lipid research in P. patens remains a niche topic, though the organism contains like other bryophytes some interesting differences in lipid composition compared to seed plants. Recently, the liverwort Marchantia polymorpha has emerged as new bryophyte model organism (Ishizaki et�al. 2016), which could also lead to insights into moss lipid composition in the near future. Though specific research into lipid composition of bryophytes is still limited, it can be assumed that the unique features of these early plants are connected to their lipid composition as well (Dembitsky 1993). Mosses make up substantial parts of the biomass in cold climate zones, like arctic tundra and boreal woodlands, where vascular plants are far less viable (R�tten and Santarius 1992). Mosses can survive complete desiccation (Proctor et�al. 2007) and frozen hibernation for thousands of years (Roads et�al. 2014). The adaptation to low temperatures and reduced availability of water has been linked to lipids in vascular plants many times (Hincha and Zuther 2014, Ohlrogge et�al. 2015), since these abiotic stresses also affect agriculture a lot (Bohnert and Sheveleva 1998). Studying how bryophytes acquired their high tolerance to abiotic stresses on a level of lipid composition could also lead to valuable insights into how crop plants could be modified to deal with these conditions through genetic engineering.
In this review, we will summarize the current state of the art in lipid research of P. patens since the last comprehensive review on bryophyte lipids (Dembitsky 1993). Moreover, we will discuss first insights into the function of lipids in this moss.
Membrane Lipids
Fatty acids
FA represent the basic building block of most lipids. They consist of a long hydrocarbon chain (with variable length) and a carboxy head group at one end of the chain. Nomenclature of FA is commonly as follows: X:Y, with X representing the number of carbon atoms and Y the number of double bonds inside the carbon chain. The position of double bonds is often specified by either giving an exact position inside the carbon chain (Δ#, counting from the carboxy head group) or giving the position of the last double bond (n-#, counting from the methyl end of the carbon chain) with additional double bonds normally being located in intervals of three carbon atoms closer to the carboxy head group.
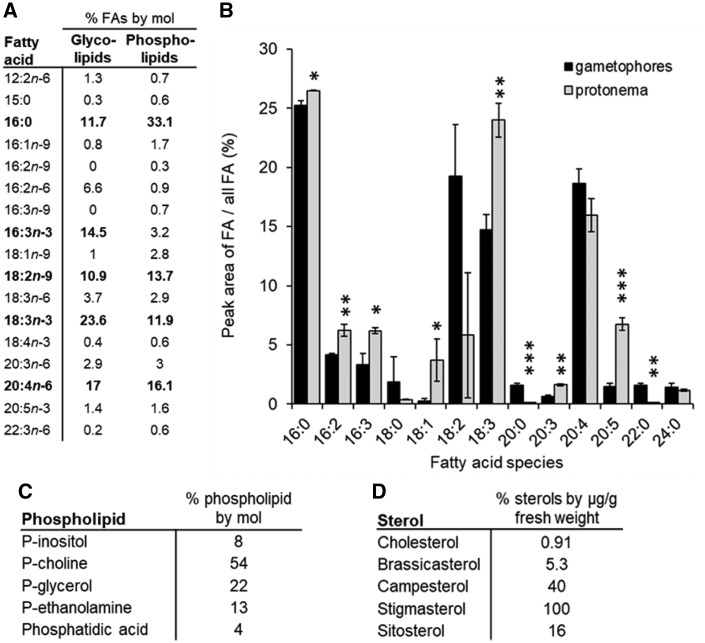
All bryophytes, including P. patens, are different from seed plants in that they contain high amounts of very-long-chain polyunsaturated FA (VLC-PUFA), harboring 20 or 22 carbon atoms. In P. patens, one of the principal PUFA in all membrane lipid classes is arachidonic acid (ARA), 20:4Δ5,8,11,14. Other major FAs in P. patens are comparable to those of seed plants, including 16:3n-3, 18:2n-6 and 18:3n-3 in glycolipids, and 16:0, 18:2n-6 and 18:3n-3 in phospholipids (Fig.�2A) (Grimsley et�al. 1981). ARA was detected in other moss species as well, alongside the even further desaturated VLC-PUFA 20:5n-3 (Dembitsky 1993). Knockout mutants of P. patens that are unable to synthesize 20:4, specifically of the Δ6-desaturase (Girke et�al. 1998), of the Δ5-desaturase (Kaewsuwan et�al. 2006), and of the Δ6-elongase (Zank et�al. 2002, Eiamsa-Ard et�al. 2013), do not exhibit any visible phenotype under normal or stressed growth conditions. VLC-PUFA are commonly also detected in microalgae (Bigogno et�al. 2002, Harwood and Guschina 2009), which could mean that VLC-PUFA in bryophytes are a remnant from their marine origin and ultimately not crucial for survival under terrestrial conditions. VLC-PUFA in bryophytes could also fulfill additional functions in nature that are not needed for mosses grown at laboratory conditions, but such theories remain speculative. Other enzymes involved in FA biosynthesis have not been described for P. patens yet but the acyl:coenzyme A synthetase (ACS) gene family has been at least fully annotated (de Azevedo Souza et�al. 2008).
Fig. 2.
Composition of various groups of membrane lipids and tissue types in P. patens. (A) FA composition in glycolipids and phospholipids of P. patens (Grimsley 1981). Bold letters mark major FA with >10% relative abundance. (B) FA profile in different tissue types of P. patens (Beike et al. 2014). Asterisks represent results of a student t-test (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Distribution of head groups in phospholipids of P. patens (Grimsley 1981). (D) Distribution of steroid core structures in P. patens (Morikawa et al. 2009).
In P. patens, the different growth stages of the gametophore (filamentous protonema and leaf-like gametophyte tissue) do not differ significantly from each other in their FA profile (Fig.�2B) (Beike et�al. 2014). There are slight differences in that gametophyte tissue contains less 18:3 and 20:5 FAs compared to protonema tissue, with a subsequent increase of 18:2 (Fig.�2B).
A first hint that the desaturation of FA may be in involved in the cold stress response in the moss as well was provided recently by identifying the cold-induced expression of a transcript for a yet unknown desaturase (Beike et�al. 2015).
Polar glycerolipids
Glycerolipids are the most common type of membrane lipids in most organisms, consisting of a glycerol backbone to which usually two FA and a polar head group are attached to. The type of polar head group defines the function and name of that specific lipid class. Phospholipids, which possess different types of head groups that all base on phosphate, are found in almost all types of membranes. Glycoglycerolipids are exclusively found in plants and make up the majority of lipids found in plastids (Ohlrogge et�al. 2015).
Research on molecular composition of glycerolipids in P. patens is scarce, being limited to mostly FA profiles of broad lipid classes, like glycolipids, phospholipids and neutral lipids (Grimsley et�al. 1981). Phospholipids were analyzed a degree more thoroughly, separating contents of different phospholipid subclasses (Fig.�2C) (Grimsley et�al. 1981). Physcomitrella patens phospholipids are mostly composed of phosphatidyl(P)-choline (54%) and P-glycerol (22%), and lesser amounts of P-ethanolamine, P-inositol and phosphatidic acid. This is largely similar to what was found for A. thaliana, with the main difference that P-choline and P-glycerol are present in A. thaliana in about equal amounts (Welti et�al. 2002). However, other publications found that P-ethanolamine is present in higher amounts in A. thaliana lipids (Browse et�al. 1986).
Other lipid classes were not analyzed in extent, however, it is known that the glycolipid class monogalactosyl-diacylglycerol and betaine lipids are present in other classes of bryophytes (Dembitsky 1993). Betaines are zwitterionic non-phosphorous lipids with similar functions as phospholipids and are known from marine microalgae to play a major role in the composition of lipids (Eichenberger et�al. 1993, Ca�avate et�al. 2016). The presence of these algae-typical lipids in bryophytes could be attributed to the fact these plants are still partially adapted to living under conditions that marine organisms favor.
Sterols
Sterols represent a lipid class that is not formed from FA but from isopentenyl pyrophosphate instead. As in all eukaryotes, sterols in plants are necessary for survival (Ohlrogge et�al. 2015). As part of the plasma membrane, they are important for establishing membrane fluidity in concert with other lipid classes. In P. patens, free sterols were analyzed (Morikawa et�al. 2009), with stigmasterol being the most prominent (Fig.�2D). Other major sterols are campesterol (40%) and sitosterol (16%), brassicasterol and cholesterol are only present in minor amounts. It is noteworthy that in vascular plants, the sterol species sitosterol is only present in minor amounts (Schaeffer et�al. 2001), while it was detected in all analyzed bryophytes so far (Dembitsky 1993). Complex sterols (glycosylated or acylated) were not analyzed in P. patens to this date.
Sphingolipids
Sphingolipids represent a complex lipid class with a variety of confirmed and assumed functions in plants (Markham et�al. 2013). In general, sphingolipids are only scarcely studied in all bryophytes. The sphingolipid class glycosyl-inositol-phosphoryl ceramide, which is common in vascular plants like A. thaliana (Markham and Jaworski 2007), was detected in a comparative study of different plant species (Bur� et�al. 2014), but no molecular species analysis was performed till now. There has no other study on sphingolipids in P. patens been published to date.
Storage Lipids and Lipid Droplets
In vascular plants, neutral lipids serve as storage molecules mostly in seeds. These storage lipids consist mainly of lipids without polar head groups, such as triacylglycerols and sterol esters. The exact composition of sterol esters or triacylglycerols has to this day not been analyzed in P. patens, but it is known that bryophytes in general can contain a relatively high amount of triacylglycerols in green tissues, especially compared to vascular plants. In some bryophytes, like Ceratodon purpureus, the overall lipid content can be composed of more than 70% triacylglycerols (Dembitsky 1993). However, it is not clear if P. patens can have a similar makeup of storage lipids.
Storage lipids are deposited in eukaryotes in so-called oil bodies or lipid droplets (LD). They are surrounded by a monolayer of membrane lipids, which also inhabit several specific types of proteins (Chapman et�al. 2012, Huang 2018). In plants, LD can be found in all tissue types, but accumulate in seed and pollen. LD in seeds are also defined by the presence of oleosins, the most abundant type of LD specific proteins, which are commonly only found in this type of tissue (Chapman et�al. 2012, Huang 2018). The research on LD of P. patens, as for all bryophytes, is limited. It was however discovered that LD from P. patens vegetative gametophyte tissue contains oleosins (Huang et�al. 2009), hinting towards some discrepancy between LD of vascular plants and bryophytes. Physcomitrella patens was also the evolutionary most ancient plant reported to contain oleosins (Huang et�al. 2009), since these types of proteins have so far not been reported in marine plants like microalgae. The composition of lipids in the LD of P. patens is mostly similar to that of A. thaliana vegetative tissue, containing mostly triacylglycerols and sterol esters, but no molecular analysis of these lipids has been conducted so far (Huang et�al. 2009).
Surface Lipids
Bryophytes are concluded to be the first land plants, thus they had to adapt to live in non-aqueous environment. To protect themselves from desiccation, UV radiation and pathogens they developed a hydrophobic protective surface layer—the cuticle. Although some green algae, like Coleochaete, are covered with a hydrophobic cuticle-like layer, bryophytes are the first organisms that developed a cuticle similar to that of flowering plants (Cook and Graham 1998, Yeats et�al. 2014).
The aerial parts of P. patens—gametophores and spores—are covered with a cuticle, composed of a very hydrophobic polymer cutin and cuticular waxes (Buda et�al. 2013). In flowering plants, cutin is mostly built of hydroxyl FA and of small amounts of phenyl compounds. They are linked together via ester bonds, forming the scaffold of the cuticle (Franke et�al. 2005, Fich et�al. 2016). Waxes are a group of components such as alkanes, aldehydes, saturated FA, ketones, fatty alcohols and wax esters which are covering and sealing the cutin and consequently the plant surface (Rashotte et�al. 2001, Samuels et�al. 2008). Cutin as well as waxes are synthesized in the ER and afterwards transported through the cell wall to cover the plant surface (Kunst and Samuels 2003). Many studies considering cuticle structure and biosynthesis were performed in seed plants, especially in A. thaliana, but relatively little is known about this structure in mosses.
Cutin
Cutin of P. patens is very rich in phenolic compounds, especially in m- and p-coumaric and caffeic acid, but does however not contain ferulic acid, the most abundant phenolic compound found in the cutin of A. thaliana (Riley and Kolattukudy 1975, Rautengarten et�al. 2012, Buda et�al. 2013). An enzyme catalyzing the formation of caffeic acid was characterized as a cytochrome P450 belonging to CYP98 family (PpCYP98) (Renault et�al. 2017). Enzymes from the CYP98 family, in higher plants, are involved in the phenylpropanoid pathway, a source of metabolites required for lignin biosynthesis (Schoch et�al. 2006, Fraser and Chapple 2011). Mutants of PpCYP98 were showing stunted growth and aborted development of gametophores. This can be the result of organ fusion caused by a low cutin content and a lack of caffeic acid (Renault et�al. 2017). As mentioned, the CYP98 enzyme family is involved in biosynthesis of major monolignols that are not present in bryophytes. It was therefore proposed that the pre-lignin pathway is crucial for cuticle formation in P. patens. This biosynthesis pathway leading to a high amount of phenolic compounds, which are important for the cuticle structure of P. patens, suggests that there is a common ancestor of the formation of cutin, lignin and also suberin, the latter one being a polymer rich in phenolic compounds present only in seed plants (Renault et�al. 2017). This ancestral structure could be the first and critical hydrophobic layer necessary for the transition from a water to a land environment. Another example of plants adapting to land conditions is sporopollenin, a polymer that is covering the pollen of flowering plants (Piffanelli et�al. 1998, Ariizumi and Toriyama 2011). Despite the fact that sporopollenin was not found in P. patens, an enzyme involved in its biosynthesis (the type III polyketide synthase—PpORS) was recently characterized in this organism (Li et�al. 2018). It is closely related to FA synthases involved in cutin and wax biosynthesis in seed plants. Mutants of PpORS showed malformed phyllids (leaf-like structures) and could not recover after desiccation. It was suggested that mutants of PpORS have a compromised or defective cuticle and it may therefore be that the sporopollenin synthesis pathway also evolved from the same ancestor as the cuticle pathway (Li et�al. 2018).
Waxes
In P. patens, not only the composition of cutin, but also of waxes differs from that of seed plants. Wax extracted from gametophores of P. patens contains very high amounts of fatty alcohols and wax esters, whereas in seed plants the most abundant group are alkanes (Buda et�al. 2013). In P. patens none of the enzymes involved in wax biosynthesis were characterized, but one of the wax transporters, the ATP-binding cassette transporter PpABCG7, was described. PpABCG7 is a homolog of a previously characterized ABCG12 transporter in A. thaliana (Buda et�al. 2013). Mutants of PpABCG7 formed smaller gametophores which contained less wax but the same amount of cutin and their sporophytes exhibited stunted growth. However, complementation of the A. thaliana mutant abcg12 with PpABCG7 was not successful, suggesting that due to the differences in the wax composition, those two transporters might have different specificities (Buda et�al. 2013).
Physcomitrella patens has a cuticle that is in principal similar in architecture and function to that of flowering plants, however its composition differs. Those differences could be a consequence of different properties between the cuticle of P. patens and cuticle of flowering plants. The cuticle of P. patens is covering only one cell layer and may need to be more permeable for an increased gas exchange in this non-vascular plant. In addition, cutin in P. patens is highly enriched in phenolic compounds, which might be a good protection against UV radiation, one of the crucial features in land colonization. There is evidence suggesting the existence of a hydrophobic layer in first land plants being a common ancestor of the formation of cutin, suberin, lignin and also sporopollenin (Renault et�al. 2017, Li et�al. 2018). This ancient hydrophobic layer might be a crucial trait for land colonization, but its evolution and adaptation to land environment remains unknown.
Signaling Lipids
Oxylipins
Unsaturated FA can be oxidized, either through spontaneous chemical reaction or catalyzed by certain enzymes (Wasternack and Feussner 2018). The term ‘oxylipin’ describes in plants mostly those oxidized FA that derive from the reaction catalyzed by lipoxygenases (LOX). These enzymes belong to the class of non-heme dioxygenases and convert PUFA into the corresponding PUFA hydroperoxides (Liavonchanka and Feussner 2006). The hydroperoxides may then act as precursors for a variety of different oxylipin compounds (Mosblech et�al. 2009). Oxylipins can fulfill a variety of different functions, but they are usually associated with signaling and stress responses in plants (Laudert and Weiler 1998, Park et�al. 2002). The phytohormone jasmonic acid (JA), an important regulator in development and defense against herbivores in plants, directly derives from this oxylipin pathway (Vick and Zimmerman 1984, Wasternack and Hause 2013).
In contrast to membrane lipids and wax esters, oxylipins in P. patens have been described in comparatively high detail. Nevertheless, compared to vascular plants, research on bryophyte oxylipins is still limited. A survey of lipids in a great variety of mosses (not including P. patens) implies a presence of volatile compounds in bryophytes, especially fatty alcohols and aldehydes (Dembitsky 1993). A main difference between oxylipins in bryophytes and vascular plants is, similar to membrane lipids, the presence of VLC-PUFA in bryophytes, which allows consequently also a greater variety of oxylipins.
In P. patens itself, research on oxylipins has been in general more extensive and detailed than in other bryophytes. This includes the study of enzymes involved in oxylipin formation. Physcomitrella patens contains seven active variants of LOX enzymes, two of which (LOX1-2) have a high affinity towards the VLC-PUFA 20:4n-6, while the others (LOX3-7) prefer 18:3n-3 as a substrate (Senger et�al. 2005, Anterola et�al. 2009). Interestingly, similar reactions have been described later for M. polymorpha (Kanamoto et�al. 2012, Kihara et�al. 2014). The reactions catalyzed by these enzymes lead to the formation of different oxylipin species (Stumpe et�al. 2010) (Fig.�3A, B). Oxidation of 20:4 may result in the formation of two types of cleavage products, either short-chain alcohols or aldehydes of varying carbon chain length (Wichard et�al. 2005). Although the formation of the former octenols has been described in moss and fungi, their synthesis follows different pathways in these two kingdoms. While they derive from 20:4 in the moss by the activity of the described two specific LOX forms, fungi synthesize them from unsaturated C-18 FA by the action of so-called Ppo enzymes [precocious sexual inducer (psi)-producing oxygenases] which are fusion proteins that consist of two heme groups and they belong to the family of P450 enzymes (Brodhun et�al. 2010). Interestingly, the pathway found in the moss is closely related to a reaction that has been described in porcine leukocytes (Glasgow et�al. 1986). In contrast, the formation of the aldehydes follows a route known from flowering plants via the enzyme hydroperoxide lyase (HPL) which again belongs to the family of P450 enzymes (Stumpe et�al. 2006). However, for none of these short-chain oxylipins a function has been found till today, but several LOX proteins as well as the HPL have been found to be induced upon cold stress (Wang et�al. 2009).
Fig. 3.
Oxylipin metabolism in P. patens. (A) ARA-dependent LOX pathway. (B) ALA-dependent LOX pathway. (C) Preferred α-DOX reaction in P. patens. ARA, arachidonic acid (20:4n-6); HPETE, hydroperoxy eicosatetraenoic acid; EETE, epoxy-eicosatetraenoic acid; OPTA, oxo-prostatrienoic acid; ALA, α-linoleic acid (18:3n-3); HPOTE, hydroperoxy octadecatrienoic acid; EOT, epoxy octadecatrienoic acid; 12-OPDA, 12-oxo-phytodienoic acid; LOX, lipoxygenase; HPL, hydroperoxide lyase; AOS, allene oxide synthase; AOC, allene oxide cyclase; α-DOX, α-dioxygenase.
The phytohormone JA, which is found in all vascular plants, however seems to be missing from non-vascular plants, including P. patens. There are reports of JA being present in some bryophytes (Dr�bkov� et�al. 2015) and in a terrestrial alga (Hori et�al. 2014), but these findings need further evaluation and cannot yet be considered as fully established, especially since genes necessary for JA synthesis and perception were either not yet described or reportedly are not present in the organism (Hori et�al. 2014). In P. patens, the metabolic pathway for synthesizing JA is established up to the point of its plastidic precursor 12-oxo-phytodienoic acid (12-OPDA), and the enzymes allene oxide synthase (AOS) and allene oxide cyclase (AOC), which are necessary for the synthesis, are present in this organism as well (Bandara et�al. 2009, Stumpe et�al. 2010, Hashimoto et�al. 2011, Scholz et�al. 2012). In P. patens two enzymes exist that catalyze each step. While PpAOS1 is highly active with both C-18 and C-20-hydroperoxy PUFA as substrates, PpAOS2 is fully active only with C-20-substrates (Scholz et�al. 2012). Consequently, only the Ppaos1 mutant shows a significant reduction in 12-OPDA formation. Interestingly, in case of the two AOC forms a similar picture emerges. PpAOC1 is highly active with the 18:3-derived allene oxide as substrate, while PpAOC2 is fully active with both the 18:3- and 20:4-derived allene oxides (Neumann et�al. 2012). Similar findings for these enzymes were reported for other non-vascular plants (Koeduka et�al. 2015, Yamamoto et�al. 2015, Pratiwi et�al. 2017), with JA being absent in the liverwort M. polymorpha and the lycophyte Selaginella moellendorffii. Interestingly, mutations in either of the two AOC genes lead to reduced fertility and altered sporophyte morphology in P. patens (Stumpe et�al. 2010). This points to an evolutionary conserved function of cyclopentenone-derived oxylipins in non-vascular and vascular plants, since similar mutations in flowering plants lead to sterile reproductive organs (Laudert et�al. 1996, von Malek et�al. 2002, Wasternack and Hause 2013). Another approach to analyze the function of 12-OPDA was the proteomic analysis of proteins being induced upon exogenous application with this substance (Toshima et�al. 2014). This study confirmed an observation made in Arabidopsis: the biosynthesis of cyclopentenones is regulated by a feed-forward loop, because 12-OPDA treatment led to an increase in PpAOC1. In addition, first hints are published that 12-OPDA may be involved in regulating defense responses in the moss as well (Oliver et�al. 2009). The receptor COI1 which senses the active compound JA-Ile in vascular plants, however, seems to be present in non-vascular plants (Han 2017) and could interact there with other compounds deriving from OPDA. For Marchantia it has been recently shown that the C-16 derivative of 12-OPDA, dinor-OPDA is its ligand (Monte et�al. 2018). Furthermore, the authors speculate that receptor and ligand in plants co-evolved to better fit the more complex physiology of vascular plants. It remains unclear, however, in which plant organism JA first appears or if other phytohormones, like 12-OPDA, would fulfill similar functions in early non-vascular plants.
Physcomitrella patens also harbors a variant of the enzyme α-dioxygenase (α-DOX), which in vascular plants can α-oxygenate FAs (Fig.�3C) (Hamberg et�al. 2005). Products of this enzyme may be involved in regulating both developmental processes and defense responses (Machado et�al. 2015, Ponce De Le�n et�al. 2015, Alvarez et�al. 2016).
Phosphoinositides
Phosphoinositides have not only a function as membrane lipids being involved in controlling cell polarity, membrane trafficking, ion channels and the cytoskeleton, but serve in addition as signaling molecules (Heilmann and Heilmann 2015). By analyzing the role of two phosphatidylinositol phosphate kinases that phosphorylate the inositol head group leading to the formation of phosphoinositol bisphosphates, it turned out that these highly phosphorylated lipid molecules play a crucial role in the regulation of tip growth in rhizoids (Saavedra et�al. 2009, Saavedra et�al. 2011). This is similar to what has been shown for Arabidopsis before (Kusano et�al. 2008, Ischebeck et�al. 2010). Phosphoinositide-specific phospholipase C cleave these molecules again by generating two second messengers, inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. By analyzing a loss-of-function mutant of PpPLC1, its phenotype revealed a function of IP3 and/or diacylglycerol in regulating the gravity response of the moss (Repp et�al. 2004).
Concluding Remarks
Despite the growing interest in P. patens on a general scientific level, this moss model organism remains largely unmapped in terms of lipid composition, membrane structure and related fields. Much research on P. patens lipids was obtained either several decades ago, or was limited in scale to a few subgroups. However, what is now known about lipid composition of P. patens suggests some very interesting differences and similarities in the lipid compositions and functions of mosses and vascular plants on one hand and mosses and microalgae on the other. Hopefully continued research in the future on the molecular composition and tissue specific localization of lipids in P. patens will lead to further insights into the evolution of lipid synthesis and function, membrane structure and signaling from marine plants to vascular plants.
Funding
Deutsche Forschungsgemeinschaft [DFG, IRTG 2172 ‘PRoTECT’ to I.F.]; G�ttingen Graduate School of Neurosciences, Biophysics, and Molecular Biology to H.C.R., M.L. and J.G.
Disclosures
The authors have no conflicts of interest to declare.
References
- Alvarez A., Montesano M., Schmelz E., Ponce De Le�n I. (2016) Activation of shikimate, phenylpropanoid, oxylipins and auxin pathways in Pectobacterium carotovorum elicitors-treated moss. Front. Plant Sci. 7: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anterola A., G�bel C., Hornung E., Sellhorn G., Feussner I., Grimes H. (2009) Physcomitrella patens has lipoxygenases for both eicosanoid and octadecanoid pathways. Phytochemistry 70: 40–52. [DOI] [PubMed] [Google Scholar]
- Ariizumi T., Toriyama K. (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 62: 437–460. [DOI] [PubMed] [Google Scholar]
- Ashton N.W., Cove D.J. (1977) The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol. Gen. Genet. 154: 87–95. [Google Scholar]
- Bandara P.K., Takahashi K., Sato M., Matsuura H., Nabeta K. (2009) Cloning and functional analysis of an allene oxide synthase in Physcomitrella patens. Biosci. Biotechnol. Biochem. 73: 2356–2359. [DOI] [PubMed] [Google Scholar]
- Beike A.K., Jaeger C., Zink F., Decker E., Reski R. (2014) High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 33: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beike A.K., Lang D., Zimmer A.D., W�st F., Trautmann D., Wiedemann G., et al. (2015) Insights from the cold transcriptome of Physcomitrella patens: global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. New Phytol. 205: 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigogno C., Khozin-Goldberg I., Boussiba S., Vonshak A., Cohen Z. (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60: 497–503. [DOI] [PubMed] [Google Scholar]
- Bohnert H.J., Sheveleva E. (1998) Plant stress adaptations—making metabolism move. Curr. Opin. Plant Biol. 1: 267–274. [DOI] [PubMed] [Google Scholar]
- Brodhun F., Schneider S., G�bel C., Hornung E., Feussner I. (2010) PpoC from Aspergillus nidulans is a fusion protein with only one active haem. Biochem. J. 425: 553–565. [DOI] [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C.R., Slack C.R. (1986) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem. J. 235: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda G.J., Barnes W.J., Fich E.A., Park S., Yeats T.H., Zhao L., et al. (2013) An ATP binding cassette transporter is required for cuticular wax deposition and desiccation tolerance in the moss Physcomitrella patens. Plant Cell 25: 4000–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bur� C., Cacas J.L., Mongrand S., Schmitter J.M. (2014) Characterization of glycosyl inositol phosphoryl ceramides from plants and fungi by mass spectrometry. Anal. Bioanal. Chem. 406: 995–1010. [DOI] [PubMed] [Google Scholar]
- Ca�avate J.P., Armada I., R�os J.L., Hachero-Cruzado I. (2016) Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 124: 68–78. [DOI] [PubMed] [Google Scholar]
- Chapman K.D., Dyer J.M., Mullen R.T. (2012) Biogenesis and functions of lipid droplets in plants. J. Lipid Res. 53: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J.T., Warnock R.C.M., Donoghue P.C.J. (2011) Establishing a time-scale for plant evolution. New Phytol. 192: 266–301. [DOI] [PubMed] [Google Scholar]
- Cook M.E., Graham L.E. (1998) Structural similarities between surface layers of selected charophycean algae and bryophytes and the cuticles of vascular plants. Int. J. Plant Sci. 159: 780–787. [Google Scholar]
- Cove D. (2005) The moss Physcomitrella patens. Annu. Rev. Genet. 39: 339–358. [DOI] [PubMed] [Google Scholar]
- Cove D., Bezanilla M., Harries P., Quatrano R. (2006) Mosses as model systems for the study of metabolism and development. Annu. Rev. Plant Biol. 57: 497–520. [DOI] [PubMed] [Google Scholar]
- Cuming A.C. (2012) Molecular bryology: mosses in the genomic era. Field Bryol. 103: 9–13. [Google Scholar]
- de Azevedo Souza C., Barbazuk B., Ralph S.G., Bohlmann J., Hamberger B., Douglas C.J. (2008) Genome-wide analysis of a land plant-specific acyl: coenzymeA synthetase (ACS) gene family in Arabidopsis, poplar, rice and Physcomitrella. New Phytol. 179: 987–1003. [DOI] [PubMed] [Google Scholar]
- Decker E., Frank W., Sarnighausen E., Reski R. (2006) Moss systems biology en route: phytohormones in Physcomitrella development. Plant Biol. 8: 397–406. [DOI] [PubMed] [Google Scholar]
- Dembitsky V.M. (1993) Lipids of bryophytes. Prog. Lipid Res. 32: 281–356. [DOI] [PubMed] [Google Scholar]
- Dr�bkov� L.Z., Dobrev P.I., Motyka V. (2015) Phytohormone profiling across the bryophytes. PLoS One. 10: e0125411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiamsa-Ard P., Kanjana-Opas A., Cahoon E., Chodok P., Kaewsuwan S. (2013) Two novel Physcomitrella patens fatty acid elongases (ELOs): identification and functional characterization. Appl. Microbiol. Biotechnol. 97: 3485–3497. [DOI] [PubMed] [Google Scholar]
- Eichenberger W., Araki S., Muller D.G. (1993) Betaine lipids and phospholipids in brown algae. Phytochemistry 34: 1323–1333. [Google Scholar]
- Engel P.P. (1968) The induction of biochemical and morphological mutants in the moss Physcomitrella patens. Am. J. Bot. 55: 438–446. [Google Scholar]
- Fich E.A., Segerson N.A., Rose J.K.C. (2016) The plant polyester cutin: biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 67: 207–233. [DOI] [PubMed] [Google Scholar]
- Frank W., Decker E.L., Reski R. (2005) Molecular tools to study Physcomitrella patens. Plant Biol. (Stuttg) 7: 220–227. [DOI] [PubMed] [Google Scholar]
- Franke R., Briesen I., Wojciechowski T., Faust A., Yephremov A., Nawrath C., et al. (2005) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66: 2643–2658. [DOI] [PubMed] [Google Scholar]
- Fraser C.M., Chapple C. (2011) The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 9: e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girke T., Schmidt H., Z�hringer U., Reski R., Heinz E. (1998) Identification of a novel Δ6-acyl-group desaturase by targeted gene disruption in Physcomitrella patens. Plant J. 15: 39–48. [DOI] [PubMed] [Google Scholar]
- Glasgow W.C., Harris T.M., Brash A.R. (1986) A short-chain aldehyde is a major lipoxygenase product in arachidonic acid-stimulated porcine leukocytes. J. Biol. Chem. 261: 200–204. [PubMed] [Google Scholar]
- Grimsley N.H., Grimsley J.M., Hartmann E. (1981) Fatty acid composition of mutants of the moss Physcomitrella patens. Phytochemistry 20: 1519–1524. [Google Scholar]
- Hamberg M., Ponce de Leon I., Rodriguez M.J., Castresana C. (2005) α-Dioxygenases. Biochem. Biophys. Res. Commun. 338: 169–174. [DOI] [PubMed] [Google Scholar]
- Han G.-Z. (2017) Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 68: 1323–1331. [DOI] [PubMed] [Google Scholar]
- Harwood J.L., Guschina I.A. (2009) The versatility of algae and their lipid metabolism. Biochimie 91: 679–684. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Takahashi K., Sato M., Bandara P., Nabeta K. (2011) Cloning and characterization of an allene oxide cyclase, PpAOC3, in Physcomitrella patens. Plant Growth Regul. 65: 239–245. [Google Scholar]
- Heilmann M., Heilmann I. (2015) Plant phosphoinositides—complex networks controlling growth and adaptation. Biochim. Biophys. Acta 1851: 759–769. [DOI] [PubMed] [Google Scholar]
- Hincha D.K., Zuther E. (2014) Plant Cold Acclimation. Springer New York, New York, NY. [Google Scholar]
- Hohe A., Rensing S.A., Mildner M., Lang D., Reski R. (2002) Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-box gene in the moss Physcomitrella patens. Plant Biol. 4: 595–602. [Google Scholar]
- Hori K., Maruyama F., Fujisawa T., Togashi T., Yamamoto N. (2014) Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 5: 3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.H.C. (2018) Plant lipid droplets and their associated proteins: potential for rapid advances. Plant Physiol. 176: 1894–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-Y., Chung C.-I., Lin Y.-C., Hsing Y.-I.C., Huang A.H.C. (2009) Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol. 150: 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck T., Seiler S., Heilmann I. (2010) At the poles across kingdoms: phosphoinositides and polar tip growth. Protoplasma 240: 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K., Nishihama R., Yamato K.T., Kohchi T. (2016) Molecular genetic tools and techniques for Marchantia polymorpha research. Plant Cell Physiol. 57: 262–270. [DOI] [PubMed] [Google Scholar]
- Kaewsuwan S., Cahoon E.B., Perroud P.-F., Wiwat C., Panvisavas N., Quatrano R.S., et al. (2006) Identification and functional characterization of the moss Physcomitrella patens Δ5-desaturase gene involved in arachidonic and eicosapentaenoic acid biosynthesis. J. Biol. Chem. 281: 21988–21997. [DOI] [PubMed] [Google Scholar]
- Kanamoto H., Takemura M., Ohyama K. (2012) Cloning and expression of three lipoxygenase genes from liverwort, Marchantia polymorpha L., in Escherichia coli. Phytochemistry 77: 70–78. [DOI] [PubMed] [Google Scholar]
- Kihara H., Tanaka M., Yamato K.T., Horibata A., Yamada A., Kita S. (2014) Arachidonic acid-dependent carbon-eight volatile synthesis from wounded liverwort (Marchantia polymorpha). Phytochemistry 107: 42–49. [DOI] [PubMed] [Google Scholar]
- Koeduka T., Ishizaki K., Mwenda C., Hori K., Sasaki-Sekimoto Y., Ohta H., et al. (2015) Biochemical characterization of allene oxide synthases from the liverwort Marchantia polymorpha and green microalgae Klebsormidium flaccidum provides insight into the evolutionary divergence of the plant CYP74 family. Planta 242: 1175–1186. [DOI] [PubMed] [Google Scholar]
- Kunst L., Samuels A.L. (2003) Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 42: 51–80. [DOI] [PubMed] [Google Scholar]
- Kusano H., Testerink C., Vermeer J.E.M., Tsuge T., Shimada H., Oka A., et al. (2008) The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert D., Pfannschmidt U., Lottspeich F., Holl�nder-Czytko H., Weiler E.W. (1996) Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol. Biol. 31: 323–335. [DOI] [PubMed] [Google Scholar]
- Laudert D., Weiler E.W. (1998) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signalling. Plant J. 15: 675–684. [DOI] [PubMed] [Google Scholar]
- Li L., Aslam M., Rabbi F., Vanderwel M.C., Ashton N.W., Suh D.Y. (2018) PpORS, an ancient type III polyketide synthase, is required for integrity of leaf cuticle and resistance to dehydration in the moss, Physcomitrella patens. Planta 247: 527–541. [DOI] [PubMed] [Google Scholar]
- Liavonchanka A., Feussner I. (2006) Lipoxygenases: occurrence, functions and catalysis. J. Plant Physiol. 163: 348–357. [DOI] [PubMed] [Google Scholar]
- Machado L., Castro A., Hamberg M., Bannenberg G., Gaggero C., Castresana C., et al. (2015) The Physcomitrella patens unique alpha-dioxygenase participates in both developmental processes and defense responses. BMC Plant Biol. 15: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.E., Jaworski J.G. (2007) Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed-phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 21: 1304–1314. [DOI] [PubMed] [Google Scholar]
- Markham J.E., Lynch D.V., Napier J.A., Dunn T.M., Cahoon E.B. (2013) Plant sphingolipids: function follows form. Curr. Opin. Plant Biol. 16: 350–357. [DOI] [PubMed] [Google Scholar]
- Monte I., Ishida S., Zamarre�o A.M., Hamberg M., Franco-Zorrilla J.M., Garc�a-Casado G., et al. (2018) Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 14: 480–488. [DOI] [PubMed] [Google Scholar]
- Morikawa T., Saga H., Hashizume H., Ohta D. (2009) CYP710A genes encoding sterol C22-desaturase in Physcomitrella patens as molecular evidence for the evolutionary conservation of a sterol biosynthetic pathway in plants. Planta 229: 1311–1322. [DOI] [PubMed] [Google Scholar]
- Mosblech A., Feussner I., Heilmann I. (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 47: 511–517. [DOI] [PubMed] [Google Scholar]
- Neumann P., Brodhun F., Sauer K., Herrfurth C., Hamberg M., Brinkmann J., et al. (2012) Crystal structures of Physcomitrella patens AOC1 and AOC2: insights into the enzyme mechanism and differences in substrate specificity. Plant Physiol 160: 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J., Browse J., Jaworski J.G., Somerville C. (2015) Lipids InBiochemistry & Molecular Biology of Plants. Edited by Buchanan B.B., Gruissem W., Jones R.L. pp. 337–400. John Wiley & Sons, Chichester, UK. [Google Scholar]
- Oliver J., Castro A., Gaggero C., Casc�n T., Schmelz E., Castresana C., et al. (2009) Pythium infection activates conserved plant defense responses in mosses. Planta 230: 569–579. [DOI] [PubMed] [Google Scholar]
- Park J.-H., Halitschke R., Kim H.B., Baldwin I.T., Feldmann K.A., Feyereisen R. (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 31: 1–12. [DOI] [PubMed] [Google Scholar]
- Piffanelli P., Ross J.H.E., Murphy D.J. (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex. Plant Reprod. 11: 65–80. [Google Scholar]
- Ponce De Le�n I., Hamberg M., Castresana C. (2015) Oxylipins in moss development and defense. Front. Plant Sci. 6: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratiwi P., Tanaka G., Takahashi T., Xie X., Yoneyama K., Matsuura H., et al. (2017) Identification of jasmonic acid and jasmonoyl-isoleucine, and characterization of AOS, AOC, OPR and JAR1 in the model lycophyte Selaginella moellendorffii. Plant Cell Physiol 58: 789–801. [DOI] [PubMed] [Google Scholar]
- Proctor M.C.F., Ligrone R., Duckett J.G. (2007) Desiccation tolerance in the moss Polytrichum formosum: physiological and fine-structural changes during desiccation and recovery. Ann. Bot 99: 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte A.M., Jenks M.A., Feldmann K.A. (2001) Cuticular waxes on eceriferum mutants of Arabidopsis thaliana. Phytochemistry 57: 115–123. [DOI] [PubMed] [Google Scholar]
- Rautengarten C., Ebert B., Ouellet M., Nafisi M., Baidoo E.E.K., Benke P., et al. (2012) Arabidopsis Deficient in Cutin Ferulate encodes a transferase required for feruloylation of ω-hydroxy fatty acids in cutin polyester. Plant Physiol. 158: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault H., Alber A., Horst N.A., Basilio Lopes A., Fich E.A., Kriegshauser L., et al. (2017) A phenol-enriched cuticle is ancestral to lignin evolution in land plants. Nat. Commun. 8: 14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing S.A., Lang D., Zimmer A.D., Terry A., Salamov A., Shapiro H., et al. (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. [DOI] [PubMed] [Google Scholar]
- Repp A., Mikami K., Mittmann F., Hartmann E. (2004) Phosphoinositide-specific phospholipase C is involved in cytokinin and gravity responses in the moss Physcomitrella patens. Plant J. 40: 250–259. [DOI] [PubMed] [Google Scholar]
- Reski R. (1998) Development, genetics and molecular biology of mosses. Bot. Acta 111: 1–15. [Google Scholar]
- Riley R.G., Kolattukudy P.E. (1975) Evidence for covalently attached p-coumaric acid and ferulic acid in cutins and suberins. Plant Physiol. 56: 650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roads E., Longton R.E., Convey P. (2014) Millennial timescale regeneration in a moss from Antarctica. Curr. Biol. 24: R222–R223. [DOI] [PubMed] [Google Scholar]
- R�tten D., Santarius K.A. (1992) Relationship between frost tolerance and sugar concentration of various bryophytes in summer and winter. Oecologia 91: 260–265. [DOI] [PubMed] [Google Scholar]
- Saavedra L., Balbi V., Dove S.K., Hiwatashi Y., Mikami K., Sommarin M. (2009) Characterization of phosphatidylinositol phosphate kinases from the moss Physcomitrella patens: PpPIPK1 and PpPIPK2. Plant Cell Physiol. 50: 595–609. [DOI] [PubMed] [Google Scholar]
- Saavedra L., Balbi V., Lerche J., Mikami K., Heilmann I., Sommarin M. (2011) PIPKs are essential for rhizoid elongation and caulonemal cell development in the moss Physcomitrella patens. Plant J. 67: 635–647. [DOI] [PubMed] [Google Scholar]
- Samuels L., Kunst L., Jetter R. (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 59: 683–707. [DOI] [PubMed] [Google Scholar]
- Schaefer D., Zryd J.-P., Knight C.D., Cove D.J. (1991) Stable transformation of the moss Physcomitrella patens. Mol. Gen. Genet. 226: 418–424. [DOI] [PubMed] [Google Scholar]
- Schaefer D.G., Zr�d J.P. (2001) The moss Physcomitrella patens, now and then. Plant Physiol. 127: 1430–1438. [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A., Bronner R., Benveniste P., Schaller H. (2001) The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by STEROL METHYLTRANSFERASE 2; 1. Plant J. 25: 605–615. [DOI] [PubMed] [Google Scholar]
- Schoch G.A., Morant M., Abdulrazzak N., Asnaghi C., Goepfert S., Petersen M., et al. (2006) The meta-hydroxylation step in the phenylpropanoid pathway: a new level of complexity in the pathway and its regulation. Environ. Chem. Lett. 4: 127–136. [Google Scholar]
- Scholz J., Brodhun F., Hornung E., Herrfurth C., Stumpe M., Beike A., et al. (2012) Biosynthesis of allene oxides in Physcomitrella patens. BMC Plant Biol. 12: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger T., Wichard T., Kunze S., G�bel C., Lerchl J., Pohnert G., et al. (2005) A multifunctional lipoxygenase with fatty acid hydroperoxide cleaving activity from the moss Physcomitrella patens. J. Biol. Chem. 280: 7588–7596. [DOI] [PubMed] [Google Scholar]
- Stumpe M., Bode J., G�bel C., Wichard T., Schaaf A., Frank W., et al. (2006) Biosynthesis of C9-aldehydes in the moss Physcomitrella patens. Biochim. Biophys. Acta 1761: 301–312. [DOI] [PubMed] [Google Scholar]
- Stumpe M., G�bel C., Faltin B., Beike A.K., Hause B., Himmelsbach K., et al. (2010) The moss Physcomitrella patens contains cyclopentenones but no jasmonates: mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol. 188: 740–749. [DOI] [PubMed] [Google Scholar]
- Toshima E., Nanjo Y., Komatsu S., Abe T., Matsuura H., Takahashi K. (2014) Proteomic analysis of Physcomitrella patens treated with 12-oxo-phytodienoic acid, an important oxylipin in plants. Biosci. Biotechnol. Biochem. 78: 946–953. [DOI] [PubMed] [Google Scholar]
- Vick B.A., Zimmerman D.C. (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol. 75: 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Malek B., van der Graaff E., Schneitz K., Keller B. (2002) The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 216: 187–192. [DOI] [PubMed] [Google Scholar]
- Wang X., Yang P., Zhang X., Xu Y., Kuang T., Shen S., et al. (2009) Proteomic analysis of the cold stress response in the moss, Physcomitrella patens. Proteomics 9: 4529–4538. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Feussner I. (2018) The oxylipin pathways: biochemistry and function. Annu. Rev. Plant Biol. 69: 363–386. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111: 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti R., Li W., Li M., Sang Y., Biesiada H., Zhou H.E., et al. (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 277: 31994–32002. [DOI] [PubMed] [Google Scholar]
- Wichard T., G�bel C., Feussner I., Pohnert G. (2005) Unprecedented lipoxygenase/hydroperoxide lyase pathways in the moss Physcomitrella patens. Angew. Chem. Int. Ed. Engl. 44: 158–161. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Ohshika J., Takahashi T., Ishizaki K., Kohchi T., Matusuura H., et al. (2015) Functional analysis of allene oxide cyclase, MpAOC, in the liverwort Marchantia polymorpha. Phytochemistry 116: 48–56. [DOI] [PubMed] [Google Scholar]
- Yeats T.H., Huang W., Chatterjee S., Viart H.M.F., Clausen M.H., Stark R.E., et al. (2014) Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants. Plant J. 77: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zank T.K., Zahringer U., Beckmann C., Pohnert G., Boland W., Holtorf H., et al. (2002) Cloning and functional characterisation of an enzyme involved in the elongation of Δ6-polyunsaturated fatty acids from the moss Physcomitrella patens. Plant J. 31: 255–268. [DOI] [PubMed] [Google Scholar]