Abstract.
Although the costs of dengue illness to patients and households have been extensively studied in endemic populations, international travelers have not been the focus of costing studies. As globalization and human travel activities intensify, travelers are increasingly at risk for emerging and reemerging infectious diseases, such as dengue. This exploratory study aims to investigate the impact and out-of-pocket costs of dengue illness among travelers. We conducted a prospective study in adult travelers with laboratory-confirmed dengue and recruited patients at travel medicine clinics in eight different countries from December 2013 to December 2015. Using a structured questionnaire, we collected information on patients and their health-care utilization and out-of-pocket expenditures, as well as income and other financial losses they incurred because of dengue illness. A total of 90 patients participated in the study, most of whom traveled for tourism (74%) and visited countries in Asia (82%). Although 22% reported hospitalization and 32% receiving ambulatory care while traveling, these percentages were higher at 39% and 71%, respectively, after returning home. The out-of-pocket direct and indirect costs of dengue illness were US$421 (SD 744) and US$571 (SD 1,913) per episode, respectively, averaging to a total out-of-pocket cost of US$992 (SD 2,052) per episode. The study findings suggest that international travelers incur important direct and indirect costs because of dengue-related illness. This study is the first to date to investigate the impact and out-of-pocket costs of travel-related dengue illness from the patient’s perspective and paves the way for future economic burden studies in this population.
INTRODUCTION
The past five decades have seen an unprecedented emergence of epidemic dengue and other arboviral diseases resulting from the triad of the modern world: urbanization, globalization, and human mobility.1 The beginning of the twenty-first century has marked an exponential growth in long-haul travel, which includes increased travel to dengue-endemic countries, and this trend is anticipated to extend into the future.2 Infected travelers can introduce dengue into non-endemic areas and contribute to its global spread.3–11 With climate change–associated alterations in the geographic distribution of Aedes mosquitoes,12 the risk of dengue introduction into Europe and North America has become a reality, as exemplified by the 2012 dengue outbreak in Madeira, Portugal,6 although the overall risk of establishment of dengue in Europe was modeled to be low.13 Large mass gathering events in dengue-endemic countries, such as the 2014 Fédération Internationale de Football Association World Cup and the Carnival in Brazil, now receive international attention for posing a potential health threat to travelers and to populations in non-endemic countries.14,15
Despite the increased ability in risk communication through global risk maps16 and pretravel advice on personal protective measures against mosquito bites,17,18 dengue preventive measures for travelers are limited, and dengue has emerged as a major health threat for international travelers.19 Indeed, the proportion of dengue cases in ill-returned travelers has increased markedly over the past few decades,20 and dengue is the second most commonly diagnosed febrile illness after malaria.21,22 Studies from the GeoSentinel Surveillance Network in travelers with 60 globally distributed clinics on all continents have found that dengue is the leading cause of febrile illness among ill travelers returning from Southeast Asia and Latin America and the Caribbean,23–25 and that the proportion of unwell travelers presenting to GeoSentinel sites who are found to have dengue increases from non-epidemic to epidemic years.22 Moreover, an alarming increase in dengue attack rates has been observed in European travelers.26,27 Dengue affects not only tourists8,9,28–30 but also business travelers,31 expatriates,32,33 those visiting friends and relatives,34–37 pediatric travelers,38–40 South–South travelers,41 and migrants.42–44
Infection with any of the four dengue virus serotypes can result in a wide spectrum of clinical manifestations, ranging from asymptomatic and pauci-symptomatic mild febrile illness to severe and fatal hemorrhagic fever and shock.45 Although usually a self-limiting condition lasting about 1 week and rarely fatal, dengue in travelers is a common reason for hospitalization25 and can be incapacitating.46,47 A tripling of hospitalized dengue cases was reported between 2000 and 2007 in ill-returned travelers in the United States.48 Recuperation can take several weeks because of physical weakness and depression.49 Rare complications of dengue, such as optic neuritis50 and hemophagocytic syndrome,51 have also been reported in travelers.
The impact and costs of dengue illness to patients and households have been extensively studied in dengue-endemic populations52–55; however, similar studies have never been performed in international travelers. Yet, the United Nations World Tourism Organization projected that about 1.8 billion travelers will cross international borders by 2030, with Asia and the Pacific region, where arboviral infections are endemic, highlighted as the fastest growing tourism regions in the world.56 Dengue illness can pose a considerable financial burden on international travelers because of the costs incurred while seeking and receiving medical care at destination and home country, financial losses resulting from disruption to travel itineraries of travelers and accompanying persons, and income losses if the disease persists after travelers return home. Understanding the financial burden of dengue in travelers is also important in considering the use of dengue vaccination.57–59 Within the context of a European Union funded research project on dengue,60,61 we conducted a prospective study to investigate and characterize the impact and out-of-pocket costs of travel-acquired dengue illness from the traveler’s perspective.
MATERIALS AND METHODS
Study design.
This prospective study was conducted in eight major travel or tropical medicine clinics located in Australia, Austria, Germany, Israel, Italy, the Netherlands, Switzerland, and the United States within the GeoSentinel and the TropNet travel medicine networks. Returning travelers presenting to a participating clinic with dengue-related illness were invited to participate in the study. Enrollment criteria required that the patient was an adult (18 years or older) with laboratory-confirmed diagnosis (at destination or home country) and pertinent international travel history and returned to the home country no more than 8 weeks before the study enrollment date. Recruitment occurred any time during acute infection or up to 8 weeks after illness onset. The recruitment period was 24 months, from December 2013 to December 2015.
Research procedures.
We developed a structured patient questionnaire for this study, which elicited information about patient demographic and socioeconomic characteristics, recent travel history and purpose, pretravel health consultation, dengue episode characteristics, treatment-seeking behavior, health-care utilization and associated out-of-pocket expenditures at the point of service (at destination and home country), and income and other financial losses of patients and accompanying persons, as well as the impact of dengue illness episodes on work and school life. The questionnaire was self-administered and was made available to patients in their native language.
Data collection and management.
Patients who provided informed consent were given the questionnaire with a study summary information leaflet during consultation at participating clinics and were asked to complete and return the questionnaire to the same clinic during a follow-up visit or by mail using the prepaid envelope provided. Each questionnaire was reviewed for completeness and discrepancies by site coordinators in participating clinics. Data were entered into a Microsoft Access Database (2015, Microsoft Corp, Redmond, WA) and analyzed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Analytical framework and statistical analysis.
Out-of-pocket expenditures incurred by patients as a consequence of an episode of dengue illness were categorized as direct and indirect costs. Direct and indirect costs were estimated using an ingredients-based approach in which patients were asked to report their out-of-pocket spending in these cost categories during the illness episode both at destination and home country. Direct costs were further divided into two categories: 1) medical costs and 2) nonmedical costs. Direct medical costs included costs for consultation, admission and discharge, diagnostic investigations, and medications. Direct nonmedical costs were composed of costs for meals, transportation, and lodging in a non-health facility such as a hotel while seeking treatment. Indirect costs included loss of income and nonrefundable prepaid travel costs, and travel costs of accompanying persons during dengue illness. All cost information was based on self-report. The total cost associated with each dengue episode was obtained by summing direct and indirect costs per illness episode.
The total cost per dengue illness episode was estimated using three different methods to deal with the issue of missing data. First, we assumed the costs in a given cost category were directly proportional to the out-of-pocket expenditure per capita in the home country. We found the proportionality factor that provided the best fit and used this linear regression model to impute the missing values (if any) for that cost category. Second, the mean cost in each cost category was estimated by averaging over all “available” data in that category. This approach uses all the available values and is called available-case analysis; the only drawback is that estimates are computed using different sample bases according to the pattern of missing data.62 Third and the last, we confined the analysis to the set of patients with “complete” cost data and performed the analysis on a limited part of the dataset, an approach known as complete-case analysis.62 Both available-case and complete-case analyses are applied widely, but their use is recommended when the amount of missing information is limited to ensure that the results are unbiased and reliable.62 For all three methods, the total cost per dengue illness episode was estimated by summing over direct and indirect costs.
We also reported the per-patient direct costs by treatment setting (hospitalization and ambulatory care) and geographic location (destination and home country), given the complex treatment-seeking behavior observed during dengue illness in the study population. Direct, indirect, and total costs were presented as means (SD) and expressed in 2015 in U.S. dollars. All costs reported in local currency were converted to U.S. dollars using the daily exchange rate of the day the respondent filled out the questionnaire63 and then adjusted for inflation using the consumer price index for the United States if the cost incurred before 2015.64
Ethics statement.
Each participating clinic obtained ethical approval from their local internal review board before the start of the study and followed the pertinent guidelines to ensure patient confidentiality. A signed consent form was obtained from all participants. Each questionnaire was assigned a unique patient identification number. Data on patient identifiers were stored securely and separately by site coordinators and were inaccessible to unauthorized persons. Anonymized data were used in this analysis.
RESULTS
Characteristics of study participants and dengue illness episodes.
A total of 90 adult returned travelers with laboratory-confirmed dengue infection participated in the study; the median age was 31 years (range: 18–74 years), and 46% of the patients were female. The general characteristics of the study population are presented in Table 1. Overall, 58% of the patients were aged between 18 and 34 years, 32% aged between 35 and 55 years, and 10% aged 56 or older. Although more than half (59%) had a college degree or more education, 11% reported having some college degree, 6% a professional degree, and 24% no more than a high school degree. Most (59%) were employed (part-time, full-time, or self-employed), 19% reported being a student, and 13% indicated “other” for employment status. There were several patients who were unemployed,4 retired,2 and full-time homemaker1 in the study population.
Table 1.
Characteristics of a prospective cohort of 90 travelers with laboratory-confirmed dengue
| Age (years) | 31 (18–74) |
| Age group (n, %) | |
| 18–34 years | 52 (58) |
| 35–55 years | 29 (32) |
| ≥ 56 years | 9 (10) |
| Gender (n, %) | |
| Female | 41 (46) |
| Male | 49 (54) |
| Education (n, %) | |
| College degree or more | 53 (59) |
| Some college degree | 10 (11) |
| Professional degree | 5 (6) |
| High school degree or less | 22 (24) |
| Employment status (n, %) | |
| Employed (full-time, part-time, or self) | 53 (59) |
| Student | 17 (19) |
| Unemployed/retired/full-time homemaker | 7 (7) |
| Other | 13 (14) |
Most patients reported visiting countries in Asia (82%), followed by countries in Latin America (13%). In this study population, the top destination of travel was Thailand (40 travelers, 45%). A majority of the patients (68%) reported visiting only one country, 22% visiting two countries, and 10% visiting three or more countries. Almost three-quarters of the patients reported traveling for tourism (66 travelers, 74%), most of whom self-reported to be high budget travelers (40 travelers, 45%), whereas 11% were traveling to visit family and friends, 7% for business, and 8% for other reasons. The median duration of travel was 18 days (range: 3–255). The duration of travel showed some variation by main purpose for travel, as shown in Table 2. Whereas the median duration of tourism-related travel was 18 days (3–165), business travelers stayed for a shorter period of time (median: 11 days and range: 5–27).
Table 2.
Travel characteristics and travel history of study participants
| Number of countries visited (n, %) | |
| One country | 61 (68) |
| Two countries | 20 (22) |
| Three or more countries | 9 (10) |
| Region of travel* (%) | |
| Asia | 82 |
| Latin America | 13 |
| Other (North America, Pacific, and Africa) | 5 |
| Main reason for travel† (n, %) | |
| Tourism‡ | 66 (74) |
| High budget | 40 (45) |
| Low budget | 26 (29) |
| Business | 6 (7) |
| Visiting family/friends | 10 (11) |
| Other | 7 (8) |
| Duration of travel by main reason§, days (median, range) | |
| Tourism** | 18 (3–165) |
| High budget | 16 (3–43) |
| Low budget | 23 (3–255) |
| Business | 11 (5–27) |
| Visiting family/friends | 13 (9–248) |
* During one trip, several geographic regions might have been visited.
† Main reason for recent travel unknown or unascertainable for one patient.
‡ Type of tourism is based on patient self-report.
§ Travel duration unknown for one patient.
The characteristics of dengue illness episodes are presented in Table 3. The mean duration of fever was 6.2 days (SD 3.0). The patients felt most sick, on average, 2.5 days (SD 2.5) after the onset of fever. Almost all patients (98%) reported experiencing general weakness/fatigue. The other two most commonly reported symptoms were body (muscle/bone) pain (86%) and headache (72%), followed by joint pain (59%), nausea (58%), and hypersensitive skin (57%). During dengue illness, 19 (21%) patients reported being diagnosed with one or more other diseases, of whom 12 (63%) indicated receiving additional treatment while being treated for dengue. Only three patients of 90 were previously diagnosed with dengue, but none experienced severe complications.
Table 3.
Characteristics of dengue illness episodes in study participants
| Duration of fever*, days (mean, SD) | 6.2 (3.0) |
| Number of days patient felt most sick after onset of illness, days (mean, SD) | 2.5 (2.5) |
| Previously diagnosed with dengue† (n, %) | |
| Yes | 3 (3) |
| No | 85 (96) |
| Do not know | 1 (1) |
| Disease diagnosis other than dengue (n, %) | |
| Diarrhea | 5 (26) |
| Respiratory illness | 4 (21) |
| Malaria | 3 (16) |
| Febrile illness | 3 (16) |
| Dermatological illness | 2 (11) |
| Other | 9 (47) |
* Fever duration unknown for four patients.
† History of previous dengue diagnosis unknown for one patient.
A total of 43 patients (48%) reported having received travel advice before traveling, of whom just more than half (22 patients, 51%) sought advice at a travel clinic and more than one-quarter (12 patients, 28%) from a family doctor. Almost all of these patients (39 patients, 91%) reported receiving specific advice to use personal protection measures to avoid mosquito bites during consultation. Although only about half of the patients sought pretravel health consultation, the majority (70 patients, 78%) reported using personal protection measures during travel. The average spending on personal protection measures was US$23 (SD 27) in the study population.
Health-care utilization and direct out-of-pocket costs of dengue illness.
Whereas 37% of the patients reported seeking care from a medical care provider within 24 hours and 22% within 24–48 hours of onset of illness, 41% waited more than 48 hours. The majority (61%) had emergency medical evacuation insurance and paid, on average, US$66 (SD 174) for the insurance. Only four patients used this service because of dengue illness, and this small subset of patients incurred a mean out-of-pocket cost of US$204 (SD 248).
At destination country, less than a quarter of the patients (20 patients, 22%) were hospitalized because of dengue illness, with a mean hospitalization duration of 3.7 (SD 2.8) days. None required medical care in an intensive care unit. Half of these patients (10 patients, 50%) reported re-hospitalization, whereas 14 patients (70%) received ambulatory care after returning home. A total of 29 patients (32%) sought ambulatory care while traveling, and the most frequently visited ambulatory care facilities were pharmacy (59.3%) and doctor’s office (55.6%). After returning home, 12 (41%) of these patients reported hospitalization and 23 (79%) reported receiving ambulatory care.
At home country, 35 (39%) patients were hospitalized and spent a mean of 3.1 days (SD 2.2) in the hospital. Only one patient received medical care in an intensive care unit for seven nights. More than half of these hospitalized patients (19 patients, 54%) reported also receiving ambulatory care at home. The majority (64 patients, 71%) sought ambulatory care after returning home from travel, of whom 14 (22%) reported hospitalization earlier at the destination country. The most frequently visited ambulatory facilities were outpatient department at a hospital (51.2%), the doctor’s office (23.3%), and health-care clinic (20.9%).
Table 4 presents the utilization of health-care services for dengue illness in the study population. The mean number of ambulatory care visits during a dengue illness episode was 4.4 (SD 4.8) and 3.2 (SD 4.3) at destination and home country, respectively. Using simple regression imputation, the direct medical and non-medical costs of dengue illness were estimated at US$260 (SD 553) and US$161 (SD 498) per episode, respectively, averaging to a direct out-of-pocket cost of US$421 (SD 744) per dengue illness episode.
Table 4.
Utilization of health-care services for dengue illness by international travelers
| Health-care utilization | Destination country | Home country |
|---|---|---|
| Hospitalized (n, %) | 20 (22) | 35 (39) |
| Duration of hospitalization (mean, SD) | 3.7 (2.8) | 3.1 (2.2)* |
| Visited ambulatory care facility (n, %) | 29 (32)† | 64 (71)‡ |
| Number of ambulatory care visits (mean, SD) | 4.4 (4.8) | 3.2 (4.3) |
* Duration of hospitalization unknown for one patient.
† Visit of ambulatory care facility at destination country unknown for one patient.
‡ Visit of ambulatory care facility at home country unknown for two patients.
Table 5 presents the per-patient direct costs of dengue illness in travelers as per treatment setting and geographic location. At destination country, the mean direct medical and non-medical costs of hospitalization per patient were US$461 (SD 816) and US$152 (SD 202), respectively, averaging to a direct hospitalization cost of US$592 (SD 901) per patient. Patients reported spending, on average, US$169 (SD 282) and US$61 (SD 216) for ambulatory care and transportation, respectively, totaling to a mean ambulatory care cost of US$230 (SD 408) per patient. At home country, complete out-of-pocket expenditures during hospitalization were available for only 27 patients. The mean direct and non-medical costs of hospitalization were US$24 (SD 69) and US$171 (SD 496) per patient, respectively, averaging to a total hospitalization cost of US$195 (SD 556) per patient. A total of 56 of 64 patients reported out-of-pocket expenditures for ambulatory care at home country fully. Patients spent, on average, US$134 (SD 394) for ambulatory care and US$79 (SD 49) for transportation, totaling at a mean ambulatory care cost of US$213 (SD 613) per patient.
Table 5.
The per-patient direct out-of-pocket costs of dengue illness by treatment setting and geographic location, 2015 (all costs are expressed in U.S. dollars)
| Direct costs | Destination country | Home country |
|---|---|---|
| Hospitalization (mean, SD) | N = 20 patients | N = 27 patients |
| Medical | 461 (816) | 24 (69) |
| Nonmedical | 152 (202) | 171 (496) |
| Total direct cost | 592 (901) | 195 (556) |
| Ambulatory care (mean, SD) | N = 29 patients | N = 56 patients |
| Medical | 169 (282) | 134 (394) |
| Nonmedical | 61 (216) | 79 (46) |
| Total direct cost | 230 (408) | 213.1 (623) |
Overall impact and indirect out-of-pocket costs of dengue illness.
Whereas 12 patients (14%) reported cutting back on travel for a median of 7 days (range: 1–180) because of dengue illness, seven patients (8%) had to postpone returning home for a median of 8 days (range: 1–63). The great majority of the patients (63 patients, 72%) reported impact on work and school life. Overall, each dengue episode resulted in a mean loss of 8.2 (SD 5.2) work or school days, whereas 39 patients (45%) received a mean of 8.3 (SD 4.6) paid work days off. Overall, 18 patients (20%) reported loss of income because of dengue illness, 14 patients (17%) reported incurring non-refundable travel costs, and only eight patients (9%) were accompanied by a family member or friend during illness episode and incurred additional travel-related costs. Under regression imputation, the indirect out-of-pocket cost of dengue illness averaged US$595 (SD 1,908) per episode.
Total out-of-pocket cost of dengue illness.
Out-of-pocket hospitalization costs were missing for eight patients, out-of-pocket ambulatory costs for eight patients, and income loss for two patients. In total, 17 of 90 patients were missing some cost data. Using imputation to infer the missing costs, the mean total out-of-pocket cost of dengue illness was estimated at US$992 (SD 2,052) per episode (Table 6). Under available-case analysis, the estimated mean total cost was US$993 (SD 2,079) per episode. When we included only the patients with complete cost data (n = 73), the mean total cost was estimated at US$889 (SD 2,014) per episode. Given the highly heterogenous cost data and the small number of missing cost values, we could not test whether the missing data are randomly distributed (a necessary condition for the validity of available and complete-case analysis62). However, the fact that all three estimated means were similar increased our confidence in the results. Table 6 also presents the direct, indirect, and total costs of dengue illness per episode by type of care received. Whereas the direct, indirect, and total costs were similar for patients who sought ambulatory care only (US$253, SD 422) and hospitalization only (US$254, SD 574), the total out-of-pocket expenditure was significantly and unsurprisingly higher when patients received both ambulatory and hospital care due to dengue illness (US$992, SD 2,052).
Table 6.
Out-of-pocket health expenditure for dengue illness in international travelers by treatment setting, 2015 (all costs are expressed in U.S. dollars)
| Out-of-pocket costs | Ambulatory care only (N = 37 patients)(mean, SD) | Hospitalization only (N = 12 patients)(mean, SD) | Ambulatory care and hospitalization (N = 90 patients)(mean, SD) |
|---|---|---|---|
| Direct cost | 182 (313) | 141 (475) | 421 (744) |
| Indirect cost | 70 (196) | 113 (216) | 571 (1,913) |
| Total cost | 253 (422) | 254 (574) | 992 (2,052) |
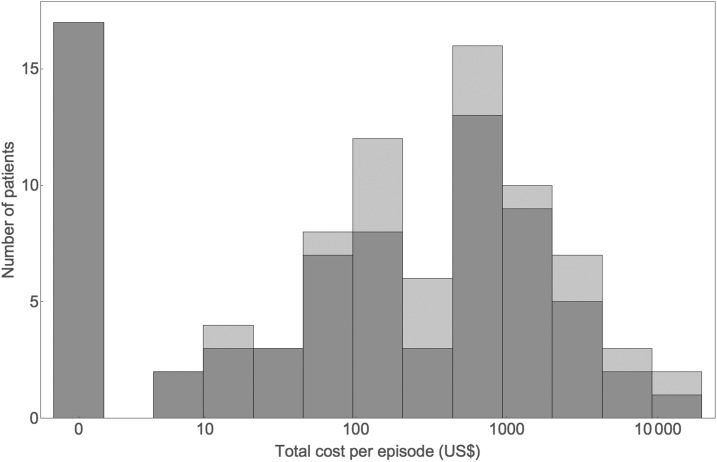
Figure 1 depicts the distribution of total out-of-pocket costs for individual patients, with the estimates using imputation and complete-case analysis overlaid. The distribution has a spike at zero, reflecting some patients reporting no costs for dengue illness. If plotted on a linear scale, the distribution exhibits a long right-hand tail. Both features are typical of cost data.65 As a result of the heavy tail, the SD is roughly twice the mean, with most costs below the mean and a few much higher. Plotted on a logarithmic scale (as in Figure 1), apart from the spike at zero, the distribution looks roughly lognormal.
Figure 1.
Distribution of total out-of-pocket costs per dengue illness episode for individual patients, with the estimates using imputation (purple) and complete-case (gray) analysis overlaid (all costs are expressed in 2015 U.S. dollars).
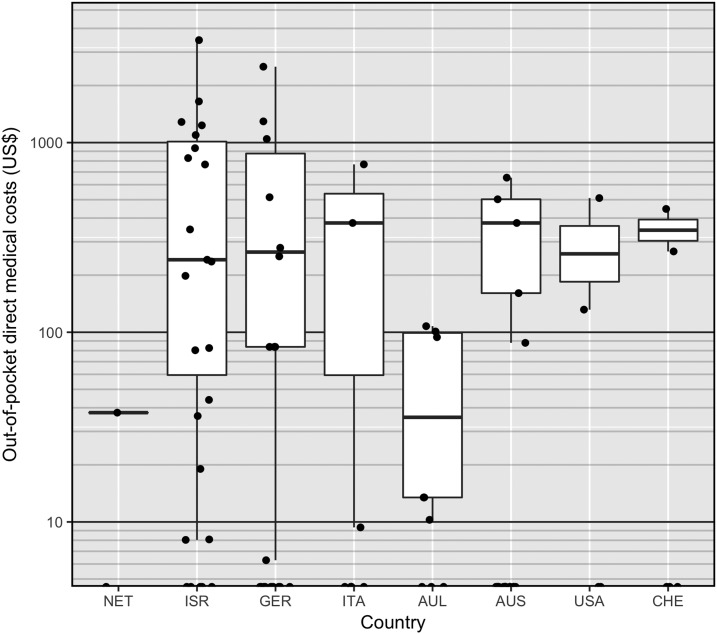
Figure 2 displays the direct out-of-pocket medical cost per dengue illness episode in each study country. The countries are ordered on the x axis by their 2015 out-of-pocket expenditure per capita, where the Netherlands (US$649) had the lowest and Switzerland (US$2,136) had the highest per capita expenditure. The solid black lines indicate the median of non-zero costs, the boxes display the interquartile range, and the whiskers extend to the lowest and highest values that are within 1.5 times the interquartile range of the box. This figure further illustrates the very wide range of out-of-pocket medical costs incurred by dengue patients in any given health-care setting.
Figure 2.
Box plot of direct out-of-pocket medical costs per dengue illness episode by out-of-pocket expenditure per capita spending in each study country (all costs are expressed in 2015 U.S. dollars).
DISCUSSION
This exploratory study investigated the utilization of health-care services for dengue-related illness and associated out-of-pocket health-care spending of international travelers at the point of service at destination and home country along with their income and travel-related financial losses. The average total out-of-pocket cost per episode at US$992 is non-trivial (Table 6), given that almost all patients experienced short-lived dengue illness and no complications. This mean per-illness episode cost was, however, estimated across 90 dengue patients who sought a range of health-care services at a variety of treatment settings from ambulatory care facilities to hospitals at both destination and home country with varying health-care systems. There was a large spread in total costs, with most costs below the mean and a few much higher (Figure 1). It is important to underscore that the indirect out-of-pocket cost at US$571 per dengue illness episode accounted for more than half the total cost, reflecting the substantial financial loss incurred by travelers because of changes in productive activities and travel itineraries as a consequence of dengue illness.
In comparison, a modeling study, which assessed the economic value of pretravel health consultation for malaria, estimated a total out-of-pocket cost of US$3,387 for hospitalized patients in the United States.66 This estimate was based on a range of out-of-pocket medical cost of US$0–US$5,000 for insured patients in the United States, which is a health-care setting with notably high patient cost-sharing requirements through deductibles, coinsurance, and co-payments, or similar charges for covered services, among high-income countries.66 For uninsured patients, the out-of-pocket direct cost was assumed to be the average direct medical cost to the health-care payer, which was US$25,250 in 2009.66 The substantial variations in out-of-pocket costs in any given setting, as illustrated in Figure 2, underscore the need for a careful assessment of such costs in much larger study populations by disease severity, treatment setting, type of provider, health insurance status, and health-care setting. Moreover, costing studies often vary in methodological approaches in terms of types of costs measured and how these costs are quantified, and hence, cost estimates may be inconsistent or noncomparable within and across countries and over time.67
We also reported on the per-patient direct costs incurred at the point of service by treatment setting and geographic location (Table 5). Our results indicate a level of out-of-pocket spending for health care that is not trivial either. Out-of-pocket medical spending for hospitalization and ambulatory care at destination country was high and averaged around US$592 and US$230 per patient. Larger proportions of travelers were hospitalized or received ambulatory care at home country (39%, 71%, respectively) than destination country (22%, 32%, respectively). Given that almost all patients reported no complications, it is unclear if all hospitalizations were necessary and appropriate. Although, in the home country, out-of-pocket medical spending for hospitalization was negligible (US$24 per patient), it was nontrivial for ambulatory care and averaged around US$134 per patient. This suggests overall lower cost-sharing requirements for hospital care than ambulatory care across health-care settings.68–70 Our results also showed that nonmedical costs at destination and home country contributed considerably to the direct costs of dengue illness in travelers, averaging US$152 and US$171 per patient for hospitalization, and US$61 and US$79 per patient for ambulatory care, respectively.
Despite the novelty of data presented here, this study was limited to a small sample of 90 travelers with laboratory-confirmed dengue who were recruited from an international network of travel clinics located in eight different countries. Small sample size precluded an analysis and comparison of out-of-pocket medical spending for dengue illness by home country to account for the differences in the health-care systems, which affect cost-sharing requirements for a given basket of health services across countries.68 Although most hospitalized patients reported having health insurance coverage for medical expenditures, they failed to reliably report on the received or anticipated reimbursement amount. This may be related to the factors associated with reimbursement processes depending on the type of health insurance, particularly for medical expenditures incurred abroad, including complex reimbursement requirements, lengthy processing wait times, and payment delays, all of which may even affect how many claims are successfully filed and accepted. There seems to be a gap in the published literature in these regards. We therefore reported on out-of-pocket medical expenditures incurred by travelers at the point of service, which might have overestimated the total out-of-pocket cost for dengue as some eligible medical expenses might have been later reimbursed and absorbed by third-party payers. Although potentially costly and administratively complex in high-income countries, this limitation can be overcome if patient-level data are extracted from or linked to health insurance information systems.71 It is also important to note that the payments into insurance premiums are not typically defined as out-of-pocket spending for an acute illness episode and were not captured in this study. Similar to other studies, the study relied on self-reported data, which are subject to error due to recall bias.72 However, incurred expenditures and losses by travelers for dengue illness were episodic in nature. Furthermore, the length of recall period was limited by recruiting patients who returned to their home country not earlier than 8 weeks before the study enrollment date to address this limitation, although this might have affected patients’ reporting on the reimbursement process and amount.
A final limitation of the study is that self-reporting of medical and nonmedical out-of-pocket expenditures was incomplete for some patients. We used three different methods to handle missing cost data. Complete-case analysis is the most commonly used approach, but it leads to information loss and is considered inefficient for univariate analysis, for example, estimation of means.62 Also, the approach relies on the assumption that patients with complete cost data are representative of those with missing data, which is often not the case, leading to biased estimates. Therefore, we also used available-case analysis and a simple regression imputation, which yielded similar mean total costs, increasing our confidence in the results.
Dengue disease cases among travelers have been increasing as the overall dengue burden has expanded globally. Costing studies from the patients’ perspective provide us with an understanding of the welfare loss associated with illnesses and the potential financial benefit of disease prevention. Although such studies fail to measure and value disutility (pain and suffering) associated with illness, direct medical and nonmedical expenditures and indirect costs are pivotal in elucidating patients’ willingness to pay to avoid another similar illness episode, and can be compared against the costs of preventive measures, including vaccination, targeted at this population and help with their prioritization.
Convincing at-risk populations and decision-makers, such as national and private health insurance providers and other third-party payers, of the need for prevention measures always involves presenting a cogent financial argument. In this small cohort of 90 patients, 39% reported being hospitalized at home country for an average of 3.1 days. Hospitalization costs not only vary across countries but also within a country based on hospital ownership type (e.g., for-profit, nonprofit, and public/government). Using an average cost of US$2,271 per inpatient day based on the 2015 Hospital Statistics of the American Hospital Association,73 which tracks inpatient health-care delivery in community hospitals open to the public, each dengue-related hospitalization would cost about US$7,040 per case in the United States. Using public hospital cost data, the direct medical cost of a dengue patient to the health system would be, on average, US$8,357 in Australia and US$3,490 in Italy.73 Although these are ballpark estimates derived from national averages74 and cost data based on expert opinion,75 they highlight the fact that out-of-pocket costs incurred by patients provide only a partial estimate of the societal-level economic burden of travel-acquired dengue illness. This exploratory study is the first to date to investigate the impact and out-of-pocket costs of travel-acquired dengue illness in ill-returned travelers, and will aid to develop evidence-based priorities in travel medicine practice76,77 and pave the way for future economic burden studies in this population.
Supplementary Files
Acknowledgments:
This work was funded partially by EU/FP7 through the DengueTools Consortium (grant agreement number 282589). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW, 2017. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis 17: e101–e106. [DOI] [PubMed] [Google Scholar]
- 2.Glaesser D, Kester J, Paulose H, Alizadeh A, Valentin B, 2017. Global travel patterns: an overview. J Travel Med 24: tax007. [DOI] [PubMed] [Google Scholar]
- 3.Quam MB, Sessions O, Kamaraj US, Rocklov J, Wilder-Smith A, 2016. Dissecting Japan’s dengue outbreak in 2014. Am J Trop Med Hyg 94: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quam MB, Wilder-Smith A, 2015. Importation index of dengue to determine the most probable origin of importation. J Travel Med 22: 72. [DOI] [PubMed] [Google Scholar]
- 5.Quam MB, Khan K, Sears J, Hu W, Rocklov J, Wilder-Smith A, 2015. Estimating air travel-associated importations of dengue virus into Italy. J Travel Med 22: 186–193. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Quam M, Sessions O, Rocklov J, Liu-Helmersson J, Franco L, Khan K, 2014. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill 19: 20718. [DOI] [PubMed] [Google Scholar]
- 7.Sessions OM, Khan K, Hou Y, Meltzer E, Quam M, Schwartz E, Gubler DJ, Wilder-Smith A, 2013. Exploring the origin and potential for spread of the 2013 dengue outbreak in Luanda, Angola. Glob Health Action 6: 21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masyeni S, Yohan B, Somia IKA, Myint KSA, Sasmono RT, 2018. Dengue infection in international travellers visiting Bali, Indonesia. J Travel Med 25: tay061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Molina JA, et al. 2017. 6-year review of +Redivi: a prospective registry of imported infectious diseases in Spain J Travel Med 24: tax035. [DOI] [PubMed] [Google Scholar]
- 10.Wilder-Smith A, Boggild AK, 2018. Sentinel surveillance in travel medicine: 20 years of GeoSentinel publications (1999–2018). J Travel Med 25: tay139. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths KM, Savini H, Brouqui P, Simon F, Parola P, Gautret P, 2018. Surveillance of travel-associated diseases at two referral centres in Marseille, France: a 12-year survey. J Travel Med 25: tay007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu-Helmersson J, Quam M, Wilder-Smith A, Stenlund H, Ebi K, Massad E, Rocklöv J, 2016. Climate change and Aedes vectors: 21st century projections for dengue transmission in Europe. EBioMedicine 7: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massad E, Amaku M, Coutinho FAB, Struchiner CJ, Burattini MN, Khan K, Liu-Helmersson J, Rocklöv J, Kraemer MUG, Wilder-Smith A, 2018. Estimating the probability of dengue virus introduction and secondary autochthonous cases in Europe. Sci Rep 8: 4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ximenes R, Amaku M, Lopez LF, Coutinho FA, Burattini MN, Greenhalgh D, Wilder-Smith A, Struchiner CJ, Massad E, 2016. The risk of dengue for non-immune foreign visitors to the 2016 summer Olympic Games in Rio de Janeiro, Brazil. BMC Infect Dis 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massad E, Wilder-Smith A, Ximenes R, Amaku M, Lopez LF, Coutinho FA, Coelho GE, Silva JB, Jr., Struchiner CJ, Burattini MN, 2014. Risk of symptomatic dengue for foreign visitors to the 2014 FIFA World Cup in Brazil. Mem Inst Oswaldo Cruz 109: 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jentes ES, Lash RR, Johansson MA, Sharp TM, Henry R, Brady OJ, Sotir MJ, Hay SI, Margolis HS, Brunette GW, 2016. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med 23: taw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalani T, et al. 2016. A comparison of compliance rates with anti-vectorial protective measures during travel to regions with dengue or chikungunya activity, and regions endemic for Plasmodium falciparum malaria. J Travel Med 23: taw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodyer L, Schofield S, 2018. Mosquito repellents for the traveller: does picaridin provide longer protection than DEET? J Travel Med 25 (Suppl 1): S10–S15. [DOI] [PubMed] [Google Scholar]
- 19.Wilder-Smith A, 2012. Dengue infections in travellers. Paediatr Int Child Health 32: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratnam I, Leder K, Black J, Torresi J, 2013. Dengue fever and international travel. J Travel Med 20: 384–393. [DOI] [PubMed] [Google Scholar]
- 21.Gautret P, Schlagenhauf P, Gaudart J, Castelli F, Brouqui P, von Sonnenburg F, Loutan L, Parola P; GeoSentinel Surveillance Network , 2009. Multicenter EuroTravNet/GeoSentinel study of travel-related infectious diseases in Europe. Emerg Infect Dis 15: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz E, Weld LH, Wilder-Smith A, von Sonnenburg F, Keystone JS, Kain KC, Torresi J, Freedman DO; GeoSentinel Surveillance Network , 2008. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis 14: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leder K, et al. GeoSentinel Surveillance Network , 2013. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med 158: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leder K, et al. GeoSentinel Surveillance Network , 2013. Travel-associated illness trends and clusters, 2000–2010. Emerg Infect Dis 19: 1049–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E; GeoSentinel Surveillance Network , 2007. Fever in returned travelers: results from the GeoSentinel surveillance network. Clin Infect Dis 44: 1560–1568. [DOI] [PubMed] [Google Scholar]
- 26.Rocklov J, Lohr W, Hjertqvist M, Wilder-Smith A, 2014. Attack rates of dengue fever in Swedish travellers. Scand J Infect Dis 46: 412–417. [DOI] [PubMed] [Google Scholar]
- 27.Cobelens FG, Groen J, Osterhaus AD, Leentvaar-Kuipers A, Wertheim-van Dillen PM, Kager PA, 2002. Incidence and risk factors of probable dengue virus infection among Dutch travellers to Asia. Trop Med Int Health 7: 331–338. [DOI] [PubMed] [Google Scholar]
- 28.Wilder-Smith A, Leong WY, 2018. Risk of severe dengue is higher in patients with sickle cell disease: a scoping review. J Travel Med 26: tay136. [DOI] [PubMed] [Google Scholar]
- 29.Serre N, Franco L, Sulleiro E, Rubio JM, Zarzuela F, Molero F, Tenorio A, 2015. Concurrent infection with dengue type 4 and Plasmodium falciparum acquired in Haiti. J Travel Med 22: 345–347. [DOI] [PubMed] [Google Scholar]
- 30.Lagi F, et al. 2014. Imported dengue fever in Tuscany, Italy, in the period 2006 to 2012. J Travel Med 21: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LH, et al. GeoSentinel Surveillance Network , 2018. Business travel-associated illness: a GeoSentinel analysis. J Travel Med: tax097 ( 10.1093/jtm/tax097). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim PL, et al. GeoSentinel Surveillance Network , 2012. Expatriates ill after travel: results from the geosentinel surveillance network. BMC Infect Dis 12: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuberger A, Turgeman A, Lustig Y, Schwartz E, 2016. Dengue fever among Israeli expatriates in Delhi, 2015: implications for dengue incidence in Delhi, India. J Travel Med 23: tay010. [DOI] [PubMed] [Google Scholar]
- 34.Heywood AE, Zwar N, 2018. Improving access and provision of pre-travel healthcare for travellers visiting friends and relatives: a review of the evidence. J Travel Med 25: taw003. [DOI] [PubMed] [Google Scholar]
- 35.Rowe K, Chaves N, Leder K, 2017. Challenges to providing pre-travel care for travellers visiting friends and relatives: an audit of a specialist travel medicine clinic. J Travel Med 24: tax038. [DOI] [PubMed] [Google Scholar]
- 36.Heywood AE, et al. 2016. The contribution of travellers visiting friends and relatives to notified infectious diseases in Australia: state-based enhanced surveillance. Epidemiol Infect 144: 3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnett ED, MacPherson DW, Stauffer WM, Loutan L, Hatz CF, Matteelli A, Behrens RH, 2010. The visiting friends or relatives traveler in the 21st century: time for a new definition. J Travel Med 17: 163–170. [DOI] [PubMed] [Google Scholar]
- 38.Rabinowicz S, Schwartz E, 2017. Morbidity among Israeli paediatric travellers. J Travel Med 24: tax062. [DOI] [PubMed] [Google Scholar]
- 39.Poddighe D, Bonomelli I, Giardinetti S, Nedbal M, Bruni P, 2016. Paediatric dengue fever diagnosed through parents’ epidemiologic report and preventive strategy during the acute phase of infection. J Travel Med 23: tav013. [DOI] [PubMed] [Google Scholar]
- 40.Hagmann S, Neugebauer R, Schwartz E, Perret C, Castelli F, Barnett ED, Stauffer WM; GeoSentinel Surveillance Network , 2010. Illness in children after international travel: analysis from the GeoSentinel Surveillance Network. Pediatrics 125: e1072– e1080. [DOI] [PubMed] [Google Scholar]
- 41.Olanwijitwong J, Piyaphanee W, Poovorawan K, Lawpoolsri S, Chanthavanich P, Wichainprasast P, Tantawichien T, 2017. Health problems among Thai tourists returning from India. J Travel Med 24: tax013. [DOI] [PubMed] [Google Scholar]
- 42.Heywood AE, Lopez-Velez R, 2018. Reducing infectious disease inequities among migrants. J Travel Med 26: tay131. [DOI] [PubMed] [Google Scholar]
- 43.Sadarangani SP, Lim PL, Vasoo S, 2017. Infectious diseases and migrant worker health in Singapore: a receiving country’s perspective. J Travel Med 24: tax014. [DOI] [PubMed] [Google Scholar]
- 44.Barnett ED, Weld LH, McCarthy AE, So H, Walker PF, Stauffer W, Cetron M; GeoSentinel Surveillance Network , 2013. Spectrum of illness in international migrants seen at GeoSentinel clinics in 1997–2009, part 1: US-bound migrants evaluated by comprehensive protocol-based health assessment. Clin Infect Dis 56: 913–924. [DOI] [PubMed] [Google Scholar]
- 45.Wilder-Smith A, Ooi EE, Horstick O, Wills B, 2019. Dengue. Lancet 393: 350–363. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz E, Mendelson E, Sidi Y, 1996. Dengue fever among travelers. Am J Med 101: 516–520. [DOI] [PubMed] [Google Scholar]
- 47.Jelinek T, Ericsson CD, Steffen R, 2000. Dengue fever in international travelers. Clin Infect Dis 31: 144–147. [DOI] [PubMed] [Google Scholar]
- 48.Streit JA, Yang M, Cavanaugh JE, Polgreen PM, 2011. Upward trend in dengue incidence among hospitalized patients, United States. Emerg Infect Dis 17: 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensenius M, Gundersen SG, Vene S, Bruu AL, 1997. Dengue fever imported to Norway. Serologically confirmed cases 1991–96. Tidsskr Nor Laegeforen 117: 4230–4233. [PubMed] [Google Scholar]
- 50.Ramos JM, Tello A, Alzamora A, Ramon ML, 2015. Optic neuritis in a traveler returning from Dominican Republic to Spain with dengue virus infection. J Travel Med 22: 133–135. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi K, Hikone M, Sakamoto N, Iwabuchi S, Kashiura M, Takasaki T, Fujita H, Ohnishi K, 2015. Dengue-associated hemophagocytic syndrome in a Japanese traveler: a case report. J Travel Med 22: 64–66. [DOI] [PubMed] [Google Scholar]
- 52.Tozan Y, Ratanawong P, Sewe MO, Wilder-Smith A, Kittayapong P, 2017. Household costs of hospitalized dengue illness in semi-rural Thailand. PLoS Negl Trop Dis 11: e0005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thalagala N, Tissera H, Palihawadana P, Amarasinghe A, Ambagahawita A, Wilder-Smith A, Shepard DS, Tozan Y, 2016. Costs of dengue control activities and hospitalizations in the public health sector during an epidemic year in urban Sri Lanka. PLoS Negl Trop Dis 10: e0004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepard DS, Undurraga EA, Halasa YA, Stanaway JD, 2016. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis 16: 935–941. [DOI] [PubMed] [Google Scholar]
- 55.Shepard DS, Undurraga EA, Halasa YA, 2013. Economic and disease burden of dengue in southeast Asia. PLoS Negl Trop Dis 7: e2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Tourism Organization and Global Tourism Economy Research Centre (UNTWO/GTERC) , 2017. Annual Report on Tourism Trends, 2017 Edition – Executive Summary. Madrid, Spain: UNWTO. [Google Scholar]
- 57.Wilder-Smith A, 2018. Serostatus-dependent performance of the first licensed dengue vaccine: implications for travellers. J Travel Med 25: tay057. [DOI] [PubMed] [Google Scholar]
- 58.Batchelor T, 2018. Timing of administration of dengue vaccine in travellers with a recent confirmed dengue infection. J Travel Med 25: tay092. [DOI] [PubMed] [Google Scholar]
- 59.Wilder-Smith A, et al. 2019. Deliberations of the strategic advisory group of experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis 19: e31–e38. [DOI] [PubMed] [Google Scholar]
- 60.Wilder-Smith A, et al. 2012. DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action 5: 17273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilder-Smith A, et al. 2018. Novel tools for the surveillance and control of dengue: findings by the DengueTools research consortium. Glob Health Action 11: 1549930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Little RJ, Rubin DB, 2014. Complete-case and available-case analysis, including weighting methods. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 63.Oanda Corporation , 2018. Historical Exchange Rates. Available at: https://www.oanda.com/fx-for-business/historical-rates2017. Accessed April 15, 2018. [Google Scholar]
- 64.Bureau of Labor Statistics , 2018. CPI Inflation Calculator. Available at: https://www.bls.gov/data/inflation_calculator.htm/. Accessed April 15, 2018. [Google Scholar]
- 65.Gilleskie DB, Mroz TA, 2004. A flexible approach for estimating the effects of covariates on health expenditures. J Health Econ 23: 391–418. [DOI] [PubMed] [Google Scholar]
- 66.Adachi K, et al. Global TravEpiNet Consortium , 2013. Economics of malaria prevention in US travelers to west Africa. Clin Infect Dis 58: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipscomb J, Yabroff KR, Brown ML, Lawrence W, Barnett PG, 2009. Health care costing: data, methods, current applications. Medical Care 47 (Suppl 1): S1–S6. [DOI] [PubMed] [Google Scholar]
- 68.Rice T, Quentin W, Anell A, Barnes AJ, Rosenau P, Unruh LY, van Ginneken E, 2018. Revisiting out-of-pocket requirements: trends in spending, financial access barriers, and policy in ten high-income countries. BMC Health Serv Res 18: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ridic G, Gleason S, Ridic O, 2012. Comparisons of health care systems in the United States, Germany and Canada. Mater Soc Med 24: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garel P, 2015. Out-of-Pocket Payments in Healthcare Systems in the European Union. Brussels, Belgium: European Hospital and Healthcare Federation. [Google Scholar]
- 71.Lund JL, Yabroff KR, Ibuka Y, Russell LB, Barnett PG, Lipscomb J, Lawrence WF, Brown ML, 2009. Inventory of data sources for estimating health care costs in the United States. Med Care 47 (7 Suppl 1: S127– S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Little RJ, Rubin DB, 2014. Introduction. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 73.Keiser Family Foundation , 2018. Health Costs and Budgets Indicators. Available at: https://www.kff.org/state-category/health-costs-budgets/. Accessed April 3, 2018. [Google Scholar]
- 74.IHPA , 2018. National Hospital Cost Data Collection, Public Hospitals Cost Report, Round 20 (Financial Year 2015–16) Darlinghurst, Australia: Independent Hospital Pricing Authority. [Google Scholar]
- 75.Guzzetta G, Trentini F, Poletti P, Baldacchino FA, Montarsi F, Capelli G, Rizzoli A, Rosà R, Merler S, Melegaro A, 2017. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Neglected Trop Dis 11: e0005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torresi J, Steffen R, 2017. Redefining priorities towards graded travel-related infectious disease research. J Travel Med 24: tax064. [DOI] [PubMed] [Google Scholar]
- 77.Leder K, et al. 2017. Travel medicine perspectives of select travel medicine experts practicing in the Asia-Pacific region. J Travel Med 24: tax012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.