Figure 1. Development of a custom-designed irinotecan silicasome nanocarrier.
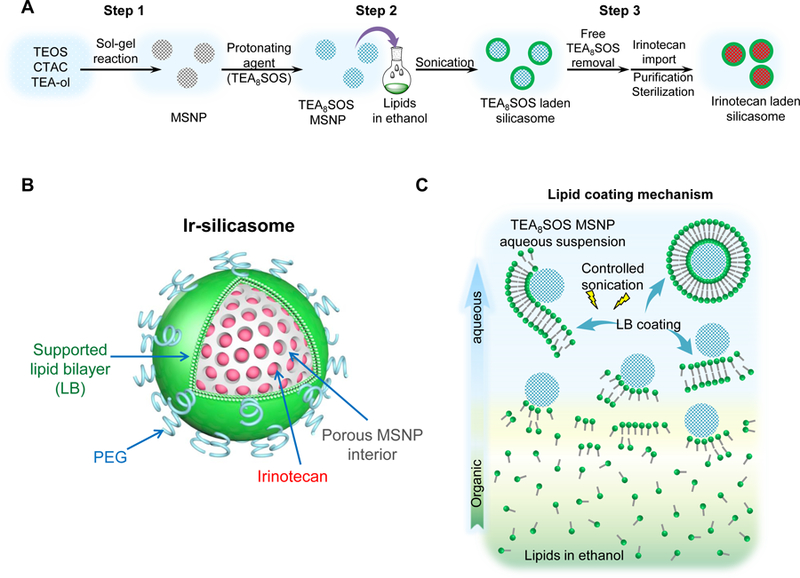
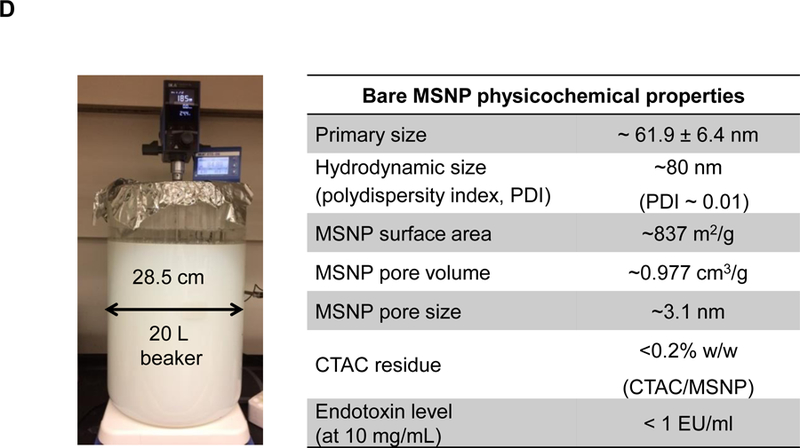
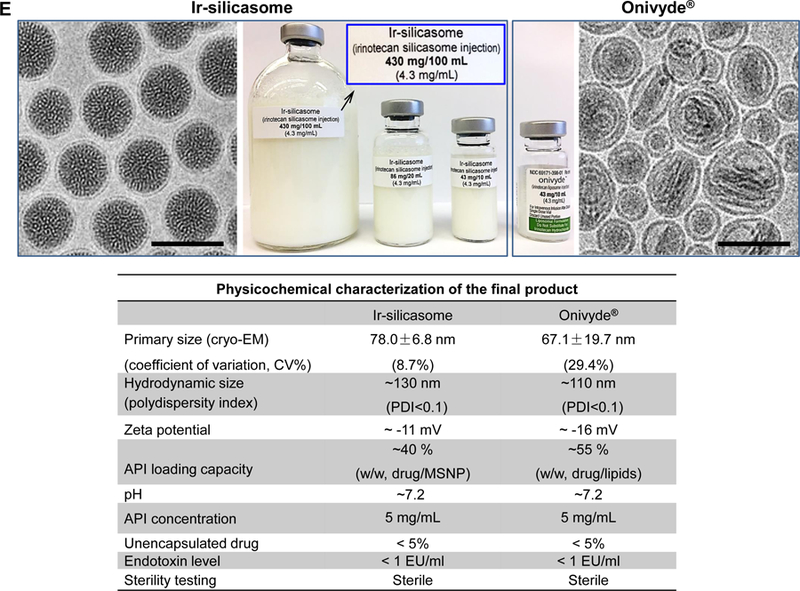
(A) Schematic to show the different steps for developing the irinotecan nanocarrier, namely: (1) bare mesoporous silica nanoparticle (MSNP) synthesis and purification (see detailed description of the high-volume process online and Figures S2-8), (2) lipid coating of the particles containing the soaked-in trapping agent, triethylammmonium sucrose octasulfate (TEA8SOS); and (3) remote loading of irinotecan by a proton gradient (generated by the trapping agent), followed by purification and sterilization. (B) The final product, the Ir-silicasome, is comprised of a MSNP core that contains a large packaging space for irinotecan, which is stably entrapped by a lipid bilayer (LB). The LB contains a PEG attachment to improve colloidal stability and circulatory half-life. (C) Schematic to show the custom-designed procedure for surface coating by an alcohol-exchange method. Lipids are dissolved in ethanol as described in the online data section Figure S1. This ethanol suspension is rapidly mixed with TEA8SOS laden particles and sonicated, which leads to the lipids assembling on the particle surface, and rapid sealing of the pores. (D) The integrated synthesis process, with precise control of temperature, stirring speed, and addition of the precursor materials at optimal ratios, is capable of producing 18 L batches that contain ~100 g of particles, as described online. The table shows the physicochemical properties of the purified bare MSNPs. (E) CryoEM visualization of the Ir-silicasome and Onivyde®. The final Ir-silicasome product contains an irinotecan (free base) concentration of 4.3 mg/mL, which was dispensed in smaller volumes in glass containers. The table summarizes the comparative physicochemical properties.
