Abstract
INTRODUCTION: The aim of the present study was to analyze the possible correlation between Natural Killer (NK) cell activity as measured by the NK Vue assay and treatment efficacy in patients with disseminated cancer. MATERIALS AND METHODS: The study included four trials encompassing palliative treatment, i.e. one trial on prostate- and ovarian cancer, respectively, and two trials on colorectal cancer. The current results are based on 93 patients with mature data on treatment effect. Blood samples were collected at baseline and prior to each treatment cycle into NK Vue. Following 24 hours of stimulation the level of interferon-gamma (IFNγ) in the plasma was measured as a surrogate for NK cell activity. RESULTS: The relationship between NK cell activity and treatment response was similar across tumor types and treatment. The IFNγ either remained at or dropped to an abnormal level (<200 pg/mL) during treatment in group 1 (n = 35). In group 2 (n = 30) the level remained within a normal range (>200 pg/mL), while in group 3 (n = 28) it increased from an abnormal to a normal level. The response rate was 14%, 47%, and 82%, respectively, P < .001. The median progression free survival was 2.6 months (95% confidence interval (CI) 2.1–3.9), 10.0 months (95% CI 6.5–11.1), and 8.3 months (95% CI 6.5–8.7), respectively, P < .001 (log-rank). CONCLUSION: Patients lacking the ability to mount an immune response during the first 2 months of treatment have a poor prognosis, and their clinical benefit of the treatment is questionable.
Introduction
Natural killer (NK) cells are part of the innate immune system and play an important role in the initial response to infected as well as malignant cells [1]. They are capable of killing target cells without prior antigen presentation and take part in coordinating the adaptive immune response through the release of inflammatory cytokines and chemokines, of which interferon-γ (IFNγ) is of great importance [1], [2].
Several studies have demonstrated debilitated functions of NK cells in patients with cancers as compared to healthy controls [3], including colorectal cancer [4], [5], [6], ovarian cancer [7], and prostate cancer [8], [9], [10]. It has also been postulated that the activity level of NK cells may provide an estimate of disease activity and relate to the escape mechanisms from immune surveillance [11]. Together, these findings designate the activity level of NK cells as a potential biomarker with implications as a tool for monitoring the immune system during treatment. More importantly, the activity level may provide clinically relevant information as to the effectiveness of a given treatment at a given time in a patient with cancer. The current standard methodologies such as 51Cr-release assays and in vitro cytokine stimulations are effective but cumbersome, which ultimately has limited the use of NK cell activity as a biomarker in the clinical setting. With the recent introduction of a simple assay for measuring NK cell activity this parameter may be clinically applicable since reliable biomarkers for treatment efficacy has been called for through decades.
The aim of the present study was to analyze the possible correlation between NK cell activity as measured by the NK Vue assay and treatment efficacy in patients with metastatic cancer.
Material and Methods
This study is reported in accordance with REMARK [12].
Patient Populations and Treatment
This translational study included patients from four ongoing biomarker studies encompassing palliative treatment in patients with metastatic disease (castration resistant prostate cancer, ovarian cancer, and colorectal cancer). The current results are based on 93 patients with mature data on treatment effect. Patients were enrolled between April 2016 and September 2017.
In brief, all patients had disseminated disease and were treated with palliative oncologic treatment according to national guidelines and protocol specifications, i.e., chemotherapy regimens in the ovarian- and colorectal cancer studies and enzalutamide in the prostate cancer study. Treatment continued until disease progression or unacceptable toxicity, but treatment-free intervals were accepted if requested by the patient. Response evaluation was based on clinical examination (including CA-125 in ovarian cancer) and CT scans of the chest and abdomen (plus bone scan in prostate cancer) every 8–12 weeks (depending on regimen) and assessed according to the RECIST 1.1 criteria [13]. Responding patients were classified as having complete response (CR) or partial response (PR), while non-responding patients were classified with stable disease (SD) or progressive disease (PD).
The main selection criteria included; histologically verified diagnosis, evaluable disease by imaging, age ≥18 years, performance status (PS) ≤2, life expectancy ≥3 months, adequate bone marrow and organ function, at least two treatment cycles received, and a minimum of two evaluations (CT scans) during treatment unless progressive disease (PD) recorded at first evaluation. The exclusion criteria were; severe medical co-morbidity, other malignant disease within 5 years prior to study enrollment, except basocellular or squamous skin cancer and carcinoma in situ cervicis uteri.
All data were recorded according to good clinical practice. The studies, and the present translational research, were approved by the Regional Committee on Health Research Ethics for Southern Denmark (S-20150185, S-20150103, S-20160029, and S-20160049) and the Danish Data Protection Agency. Written informed consent was obtained from all patients enrolled in the study.
Sampling and NK Cell Activity Analyses
Peripheral blood was sampled before initiation of treatment (baseline) and prior to each treatment cycle until disease progression. The basic principle behind the NK Vue test is the sampling of peripheral blood into a patented test tube, incubation, and estimation based on the ELISA. An illustration can be assessed at http://nkvue.com/en/sub/product/introduce.php. In specific, we did the following: One milliliter of venous blood (whole blood) was drawn from the antecubital area into NK Vue tubes (ATGen, Seongnam-si, South Korea) and placed in an incubator at 37 °C within 15 minutes of sampling. Following 24 hours of stimulation the level of IFNγ in the plasma was measured by the NK Vue ELISA (ATGen ELISA) as a surrogate marker of NK cell activity. The linear dynamic range of the ELISA was 65–2000 pg/mL, the limit of quantification was 65 pg/mL, and the limit of detection was 15 pg/mL. Samples with test results above the upper limit were diluted 1:10 and reanalyzed. Samples with activity levels below 15 pg/mL were recorded as 15 pg/mL. The in-house analytical coefficients of variation (CV) of the ELISA were <10% for Intra-Assay and <12% for Inter-Assay. A cut-off of 200 pg/mL differentiates between abnormal (<200 pg/mL) and normal (≥200 pg/mL) values (ATGen recommendation). Sampling and analyses were performed by staff unaware of patient outcome.
Prior to the study we performed duplicate analyses on 125 healthy individuals (supplementary Figure S1) indicating a valid test assay especially for test values in the range of 0–2.500 pg/mL corresponding to the majority of patients with cancer. We also tested whether the IFNγ test result corresponded to the number of NK cells. This was not the case for any of the three cancer types (supplementary Figure S2).
Statistics
We report median values with a 95% confidence interval (CI). The Wilcoxon rank sum test was used for comparison of median values. Differences between proportions were evaluated using Chi-Square Statistics. Progression free survival (PFS) was defined as the time from the first day of the first treatment cycle to the first documented tumor progression or death of any cause. The PFS data were censored in five cases due to liver surgery (patients from colorectal cancer trials only) with the date of the intervention as cut-off. Multiple comparisons were not adjusted for. All statistics were calculated using the NCSS statistical software (NCSS Statistical Software, Kaysville, UT 84037, USA, version 2007). P values <.05 were considered significant, and all tests were two-sided.
Results
Interpretation of the NK Cell Activity Test Results
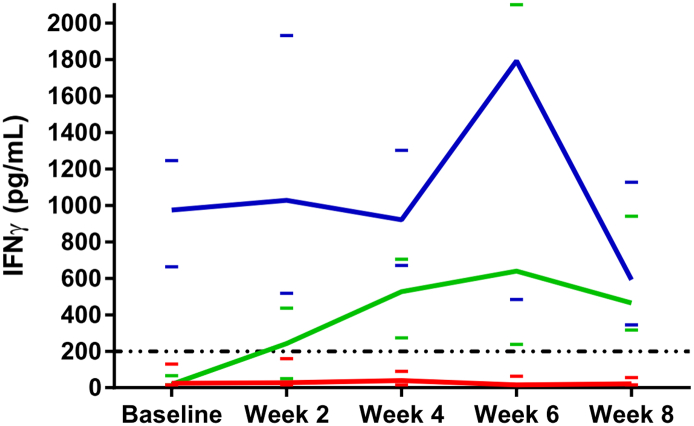
The changes in NK cell activity during the first 2 months of treatment were similar across tumor types and treatments suggesting a classification into three groups. In group 1 IFNγ dropped to an abnormal level (<200 pg/mL) or remained at an abnormal level (n = 35). The test results in group 2 (n = 30) remained within a normal range (>200 pg/mL) while patients in group 3 (n = 28) were characterized by an increase of the NK cell activity from an abnormal level at baseline to normal values. These patterns are illustrated in Figure 1. The NK cell activity in group 2 differs significantly from group 1 at all sampling points (P < .001) whereas group 3 cannot be distinguished from group 1 at baseline but diverges after only two weeks of treatment (P = .019).
Figure 1.
Illustration of median IFNγ values during the first 2 months of treatment. Group 1 is represented by the red curve, group 2 the blue, and group 3 the green curve. The dashed line marks the cut-off value of 200 pg/mL differentiating between abnormally low and normal test values. The small colored bars represent the 95% confidence intervals of the respective medians (for graphical reasons the upper limit of group 2 at week six is not depicted (7391 pg/mL)). There was no significant difference between groups 1 and 3 at baseline (P = .364), but at week 2 they were statistically different (P = .019). Group 2 differs significantly from both group 1 and 3 at baseline (P < .001 and P < .001) (Wilcoxon Rank-Sum test for difference in medians).
Patient Characteristics
The median follow-up (patients without progression) was 7.3 months and 54 patients had documented progression. Clinical and pathoanatomic characteristics are shown in Table 1. The baseline NK cell activity was significantly lower in the younger half of the patients, P = .032. Based on the fact that Group 1 and 3 cannot be distinguished from each other at baseline we compared the pathoanatomic characteristics with the division into the three groups. Table 2 shows that a Group 1 pattern was more common among the older half of the patients, prostate cancer, and patients with PS 1–2.
Table 1.
Clinical and Pathoanatomic Characteristics, n = 93
| Parameter | Combined Cohort |
Baseline NK Cell Activity (pg/mL) |
P |
|---|---|---|---|
| N = 93 (%) | Median (95% Confidence Interval) | ||
| Gender | |||
| Male | 50 (54) | 141 (34–259) | .820 |
| Female | 43 (46) | 107 (15–601) | |
| Age, median 68 | |||
| > 68 | 43 (46) | 332 (48–721) | .032 |
| ≤ 68 | 50 (54) | 53 (15–175) | |
| Line of treatment | |||
| 1 | 54 (58) | 144 (34–259) | .943 |
| 2 or more | 39 (42) | 71 (17–663) | |
| Baseline LDH, median 214 U/La | |||
| > 214 | 42 (49) | 29 (15–175) | .054 |
| ≤ 214 | 43 (51) | 199 (54–601) | |
| Disease | |||
| Colorectal cancer | 51 (55) | 126 (17–259) | .669b |
| Ovarian cancer | 23 (25) | 107 (15–663) | .335c |
| Prostate cancer | 19 (20) | 212 (20–1524) | .126d |
| Ethnicity | |||
| Caucasian | 93 (100) | 139 (31–259) | |
| Performance status | |||
| 0 | 51 (55) | 144 (39–547) | .284 |
| 1–2 | 42 (45) | 48 (15–300) | |
| Smoking statuse | |||
| Never or former smoker | 75 (86) | 142 (31–291) | .829 |
| Active smoker | 12 (14) | 47 (15–740) |
NK, Natural killer; LDH, lactate dehydrogenase.
All P values refer to the Wilcoxon Rank-Sum test for difference in medians.
a Eight patients missed values for comparisons.
b Comparison between colorectal and ovarian cancer, c comparison between ovarian and prostate cancer, d comparison between colorectal and prostate cancer.
e Data on smoking status was not available for six patients.
Bold P value highlights statistical significance.
Table 2.
Clinical and Pathoanatomic Characteristics According to the Grouping, n = 93
| Parameter | Grouping According to NK Cell Activity |
P | ||
|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||
| Gender | ||||
| Male | 22 | 12 | 16 | .167 |
| Female | 13 | 18 | 12 | |
| Age, median 68 | ||||
| > 68 | 20 | 16 | 7 | .025 |
| ≤ 68 | 15 | 14 | 21 | |
| Line of treatment | ||||
| 1 | 21 | 14 | 19 | .252 |
| 2 or more | 14 | 16 | 9 | |
| Baseline LDH, median 214 U/La | ||||
| > 214 | 20 | 10 | 16 | .084 |
| ≤ 214 | 14 | 20 | 12 | |
| Disease | ||||
| Colorectal cancer | 15 | 15 | 21 | |
| Ovarian cancer | 8 | 10 | 5 | .033 |
| Prostate cancer | 12 | 5 | 2 | |
| Performance status | ||||
| 0 | 8 | 21 | 22 | <.001 |
| 1–2 | 27 | 9 | 6 | |
| Ethnicity | ||||
| Caucasian | 35 | 30 | 28 | - |
| Smoking status | ||||
| Never or former smoker | 29 | 25 | 21 | .914 |
| Active smoker | 4 | 4 | 4 | |
NK, natural killer; LDH, lactate dehydrogenase.
P values refer to chi-square statistics.
One patient missed values for comparisons.
Treatment Efficacy
There was a significant relationship between response rate (RR) and the changes in NK cell activity during the first 2 months of treatment. The RR in group 1, 2, and 3 was 14% (5/35), 47% (14/30), and 82% (23/28), respectively, P < .001.
The association with PFS was also significant in the three groups, P < .001 (Figure 2). The median PFS was 2.6 months (95% confidence interval (CI) 2.1–3.9), 10.0 months (95% CI 6.5–11.1), and 8.3 months (95% CI 6.5–8.7), in Group 1, 2, and 3, respectively. Table 3 shows the results of the Cox regression simple and multiple analyses. Variables with P < .15 in the simple analysis were included in the multiple analysis. An independent impact on PFS was shown for the following variables; line of treatment, PS, and the NK cell activity groups. The latter demonstrated a HR of 4.368 (95% CI, 1.942–9.824), P < .001, in patients with a group 1 pattern.
Figure 2.
Kaplan–Meier progression free survival curves. Group 1 (N = 35) is represented by the red curve, group 2 (N = 30) the blue, and group 3 (N = 28) the green curve. The difference between the curves is significant, P < .001 (log-rank).
Table 3.
Cox Regression Analysis of Progression Free Survival, n = 92 in the Multiple Analysis
| Parameter | Simple Analysis |
Multiple Analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | ||||
| Female | 1 | 1 | .237 | |
| Male | 0.651 (0.378–1.120) | 0.121 | 0.634 (0.298–1.349) | |
| Age, median 68 | ||||
| ≤ 68 | 1 | |||
| > 68 | 0.913 (0.528–1.576) | 0.742 | ||
| Line of treatment | ||||
| 1 | 1 | 1 | ||
| 2 or more | 1.786 (1.038–3.075) | 0.036 | 2.450 (1.174–5.110) | .017 |
| Baseline LDH, median 214 U/La | ||||
| ≤ 214 | 1 | 1 | ||
| > 214 | 1.554 (0.884–2.734) | 0.126 | 0.943 (0.491–1.812) | .860 |
| Disease | ||||
| Colorectal cancer | 1 | |||
| Prostate cancer | 0.546 (0.237–1.258) | 0.155 | ||
| Ovarian cancer | 1.190 (0.648–2.184) | 0.575 | ||
| Smoking status | ||||
| Never or former smoker | 1 | |||
| Active smoker | 0.690 (0.309–1.543) | 0.366 | ||
| Performance status | ||||
| 0 | 1 | 1 | ||
| 1–2 | 4.340 (2.308–8.162) | <0.001 | 2.358 (1.175–4.731) | .016 |
| NK cell activity Group | ||||
| Group 3 | 1 | 1 | ||
| Group 2 | 0.770 (0.339–1.745) | 0.531 | 0.680 (0.292–1.582) | .370 |
| Group 1 | 3.614 (1.823–7.165) | <0.001 | 4.368 (1.942–9.824) | <.001 |
HR, Hazard ratio; LDH, lactate dehydrogenase; NK, natural killer.
Discussion
We analyzed the possible correlation between NK cell activity measured in the blood, by the NK Vue assay and treatment effect in patients with metastatic cancers. Changes in NK cell activity during the first 2 months of treatment were similar across tumor types and treatment and a highly significant relationship with treatment effect was demonstrated.
We have recently conducted a technical study of the NK Vue assay concluding that NK cells were the predominant source of IFNγ in the assay but T cells and natural killer T cells also contributed [14]. Having confirmed the ability of the assay to estimate NK cell activity we applied it in the clinical setting. Our present results shows that the changes in NK cell activity during the first 2 months of treatment seem to divide patients into three distinct groups regardless of tumor type and treatment. Patients with a group 1 or 3 pattern were characterized by an abnormally low NK cell activity at baseline. During the course of treatment patients in group 3 were able to mount an immune response upon stimulation while the NK cell activity in group 1 remained suppressed throughout the course of treatment. On the other hand, the immune response of patients in group 2 seemed unaffected by the disseminated cancer, thus displaying an NK cell activity within the normal range at all sampling points. The interpretation of this pattern (group 2) raises questions as to why the disease in one third of the patients appears to be unaffected by a seemingly well-functioning immune response.
The baseline NK cell activity was significantly lower in the younger patients and to some extent also low in patients with the highest baseline LDH levels, though non-significant. This observation seems plausible and may reflect 1) the often more aggressive phenotype of cancer in young patients and 2) the often greater tumor burden associated with a high level of LDH [15]. Caution should, however, be paid to the age distribution in this mixed cohort. Since the patients with prostate cancer were significantly older than the patients in the remaining three study cohorts, a disease specific impact on this comparison cannot be ruled out.
A highly significant relationship was demonstrated between the NK cell activity patterns and treatment efficacy assessed by RR and PFS. Patients in group 1 had a significantly poorer prognosis than those in the other two groups with more than half the patients progressed at first evaluation. The unfavorable outcome in these patients was further highlighted with an HR of 4.4 in the multiple Cox analysis. The treatment efficacy did not differ between groups 2 and 3.
Cancer cells may develop a variety of immune evasive strategies to escape IFNγ-dependent immune responses by downregulation of IFNγ signaling pathways, induction of signaling pathways promoting tumor growth, and production of anti-inflammatory molecules [16]. The resistance to IFNγ may be more pronounced in group 2, which could in fact explain the high test values, the modest RR, and a PFS comparable to patients in group 3.
This study is so far the first to evaluate the relationship between NK cell activity estimated by the NK Vue assay and treatment effect in patients with disseminated cancer. Consequently, a direct comparison with existing literature may be biased due to methodological differences. Similarities, however, can be drawn to a number of studies. In 2009, Ménard et al. published data on maturing dendritic cell-dependent IFNγ production by NK cells in 56 patients with gastrointestinal stromal tumors (GIST) treated with imatinib [17]. Similar to our study they assessed IFNγ levels at baseline and after 2 months of treatment. Immunological responders, defined by an increase in IFNγ above baseline values after 2 months, demonstrated a significantly better PFS compared to the non-immunological responders, i.e. decreased IFNγ values, which clearly compares to our results on group 3 and group 1 patients. Likewise, our data paralleled findings by Pasero and colleagues who demonstrated that effective NK cells in patients with newly diagnosed metastatic prostate cancer were associated with a longer time to castration resistance and overall survival [18]. Also, there were obvious similarities between our results and previous work by Garzetti et al. who followed 17 patients with ovarian cancer stage III-IV by analyzing NK cell activity at baseline (before surgery), during the course of cisplatin-containing chemotherapy, and during follow-up [19]. They demonstrated a significantly lower baseline NK cell activity in patients with subsequent disease recurrence compared to those who remained recurrence free, thus suggesting a prognostic relationship.
The modest sample size and the mixture of cancers and treatments in the current study may have influenced some of our conclusions on the relationship between NK cell activity and clinicopathological characteristics. However, the compelling relationship between test results and treatment effect substantiates the potential of this assay in different settings although the exact interpretation awaits final results from the individual tumor types.
Conclusion
The results suggest a correlation between NK cell activity and treatment effect across different solid tumor types and treatments. Patients lacking the ability to mount an immune response during the first 2 months of treatment have a poor prognosis, and the clinical benefit of the anti-cancer treatment is questionable. Assessment of NK cell activity may represent an additional way of monitoring treatment effect in oncology practice.
The following are the supplementary data related to this article.
Bland-Altman plot illustrating the difference between two consecutive measurements of NK cell activity in healthy controls (N=125). The duplicate values of IFNγ were obtained from two separate NK Vue tubes drawn immediately after each other in the same venipuncture.
Correlation between the quantity of NK cells in whole blood and IFNγ produced- and released into the plasma after 24 hours of stimulation. The coefficient of determination (R2) indicating the concentration of IFNγ only to be marginally reflected by the number of NK cells has been calculated based on a total of 40 baseline samples; 18 ovarian cancer patients, 5 prostate cancer patients, and 17 colorectal cancer patients.
Disclosure Statement
AJ has received research funding from ATGen Co. Ltd. The company had no influence on the study design, analyses, data interpretation, manuscript preparation, or submission process.
Author Contribution
All authors participated in conceiving and designing the experiments. TFH performed statistical analyses of the data and drafted the manuscript. All authors were responsible for collection and assembly of data. LN supervised the NK cell activity assessment. All authors discussed the results, commented, and approved the final version of the manuscript.
Acknowledgements
We are very thankful for the technical assistance provided by Sara Egsgaard, Lone Karlsen Jensen, Brian Thyme, and Camilla Davidsen and for the linguistic editing provided by Karin Larsen. This study was supported by ATGen, The Cancer Foundation, and the Regional Strategic Council for Research in the Region of Southern Denmark, none of which had any influence on any part of the study.
Footnotes
Support: ATGen, The Cancer Foundation, and the Regional Strategic Council for Research in the Region of Southern Denmark.
Contributor Information
Torben Frøstrup Hansen, Email: torben.hansen@rsyd.dk, torbenhansen01@hotmail.com.
Line Nederby, Email: line.nederby@rsyd.dk.
Ahmed H. Zedan, Email: ahmed.hussein.riad.zedan@rsyd.dk.
Inge Mejlholm, Email: inge.mejlholm@rsyd.dk.
Jon R. Henriksen, Email: jon.henriksen@rsyd.dk.
Karina D. Steffensen, Email: karina.dahl.steffensen@rsyd.dk.
Caroline B. Thomsen, Email: caroline.emilie.brenner.thomsen@rsyd.dk.
Louise Raunkilde, Email: louise.raunkilde.larsen@rsyd.dk.
Lars Henrik Jensen, Email: lars.henrik.jensen@rsyd.dk.
Anders Jakobsen, Email: anders.jakobsen@rsyd.dk.
References
- 1.Bryceson YT, Chiang SC, Darmanin S, Fauriat C, Schlums H, Theorell J, Wood SM. Molecular mechanisms of natural killer cell activation. J Innate Immun. 2011;3:216–226. doi: 10.1159/000325265. [DOI] [PubMed] [Google Scholar]
- 2.Gutkin DW, Shurin MR. Clinical evaluation of systemic and local immune responses in cancer: time for integration. Cancer Immunol Immunother. 2014;63:45–57. doi: 10.1007/s00262-013-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee SB, Cha J, Kim IK, Yoon JC, Lee HJ, Park SW, Cho S, Youn DY, Lee H, Lee CH. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Commun. 2014;445:584–590. doi: 10.1016/j.bbrc.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Espi A, Arenas J, Garcia-Granero E, Marti E, Lledo S. Relationship of curative surgery on natural killer cell activity in colorectal cancer. Dis Colon Rectum. 1996;39:429–434. doi: 10.1007/BF02054059. [DOI] [PubMed] [Google Scholar]
- 6.Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 2017;153:980–987. doi: 10.1053/j.gastro.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De GK, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Koo KC, Shim DH, Yang CM, Lee SB, Kim SM, Shin TY, Kim KH, Yoon HG, Rha KH, Lee JM. Reduction of the CD16(−)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastelan M, Kovacic K, Tarle R, Kraljic I, Tarle M. Analysis of NK cell activity, lymphocyte reactivity to mitogens and serotest PSA and TPS values in patients with primary and disseminated prostate cancer, PIN and BPH. Anticancer Res. 1997;17:1671–1675. [PubMed] [Google Scholar]
- 10.Barkin J, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and prostate cancer: a pilot study. Can J Urol. 2017;24:8708–8713. [PubMed] [Google Scholar]
- 11.Lin CF, Lin CM, Lee KY, Wu SY, Feng PH, Chen KY, Chuang HC, Chen CL, Wang YC, Tseng PC. Escape from IFN-gamma-dependent immunosurveillance in tumorigenesis. J Biomed Sci. 2017;24 doi: 10.1186/s12929-017-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br J Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Nederby L, Jakobsen A, Hokland M, Hansen TF. Quantification of NK cell activity using whole blood: Methodological aspects of a new test. J Immunol Methods. 2018;458:21–25. doi: 10.1016/j.jim.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Petrelli F, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Barni S. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol. 2015;54:961–970. doi: 10.3109/0284186X.2015.1043026. [DOI] [PubMed] [Google Scholar]
- 16.Kursunel MA, Esendagli G. The untold story of IFN-gamma in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81. doi: 10.1016/j.cytogfr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Menard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, Nonn C, Chaput N, Taieb J, Delahaye NF. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009;69:3563–3569. doi: 10.1158/0008-5472.CAN-08-3807. [DOI] [PubMed] [Google Scholar]
- 18.Pasero C, Gravis G, Granjeaud S, Guerin M, Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A, Olive D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360–14373. doi: 10.18632/oncotarget.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garzetti GG, Cignitti M, Ciavattini A, Fabris N, Romanini C. Natural killer cell activity and progression-free survival in ovarian cancer. Gynecol Obstet Invest. 1993;35:118–120. doi: 10.1159/000292678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bland-Altman plot illustrating the difference between two consecutive measurements of NK cell activity in healthy controls (N=125). The duplicate values of IFNγ were obtained from two separate NK Vue tubes drawn immediately after each other in the same venipuncture.
Correlation between the quantity of NK cells in whole blood and IFNγ produced- and released into the plasma after 24 hours of stimulation. The coefficient of determination (R2) indicating the concentration of IFNγ only to be marginally reflected by the number of NK cells has been calculated based on a total of 40 baseline samples; 18 ovarian cancer patients, 5 prostate cancer patients, and 17 colorectal cancer patients.