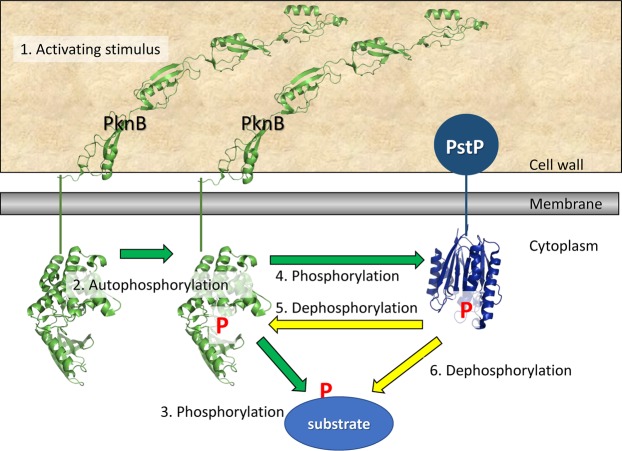
Figure 6.
Negative feedback between PknB and PstP allow a transient response to stimulation. 1 Transient stimulation of PknB. 2 PknB autophosphorylates becoming activated. 3 Activated PknB phosphorylates its substrates. 4 Activated PknB phosphorylates PstP, activating PstP. 5 Activated PstP dephosphorylates PknB, returning it to the unactivated form. 6 Activated PstP dephosphorylates PknB substrates (including itself). PknB and PstP oppose each other, so that the response to PknB stimulation is transient. When PstP is knocked down there is no negative regulator to counter the positive feedback of PknB autoactivation, so transient stimuli lead to lasting PknB activation and widespread hyperphosphorylation of PknB substrates. Structures deposited in the PDB are used in this scheme (1O6Y, 2KUI, 2CM1), although the structural changes that occur during signalling are unknown. Similar feedback loops may exist for other Mycobacterial protein kinases.