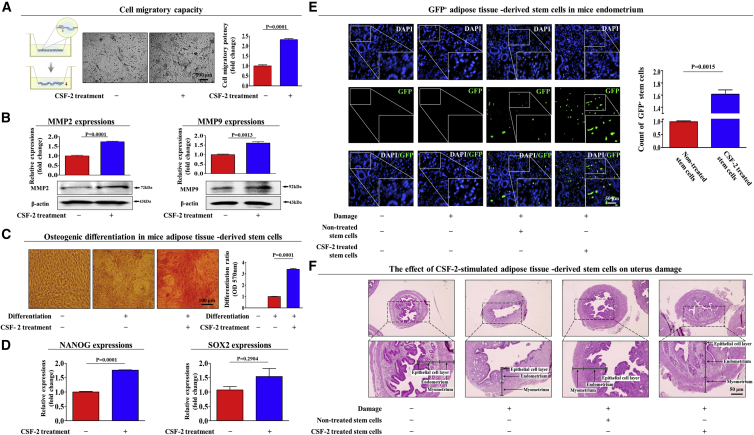
Figure 7.
CSF-2 Improves the Therapeutic Potential of MSCs by Stimulating Differentiation and Migratory Capacities in a TCA-Induced Endometrial Ablation Animal Model
Schematic representation of the experimental protocol as described in the Materials and Methods. Mice were treated daily for 10 days with CSF-2 (0.5 mg/kg, intravenously) or vehicle (PBS). Stem cells were isolated from mouse adipose tissue, and the changes in migratory capacity were measured via the transwell assay (A) and western blotting for MMP-2 and MMP-9 (B). The changes in osteoblast differentiation were determined by alizarin red staining. (C) The relative quantification of calcium mineral content was performed by measuring the absorbance at 570 nm. (D) Real-time PCR results showed the changes in the expression of the mouse stem cell markers NANOG and SOX2 after CSF-2 treatment in vivo. (E) The 2% TCA treatment (150 μL, administered directly into the uterine horn) produced significant histological uterine endometrial ablation compared with the vehicle (PBS) control. CSF-2-treated or non-treated MSCs (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old immunodeficient NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled MSCs were injected. Green fluorescent images of consecutive sections revealed the presence of GFP-labeled cells. (F) Uterine endometrial tissue was collected and subjected to H&E staining. TCA-induced loss of the endometrial functional layer with degenerative changes was significantly more relieved by the transplantation of CSF-2-pretreated MSCs. β-actin was used as the internal control. The data represent the mean ± SD from eight independent experiments.