Figure 1. Inflammasome activation and signaling.
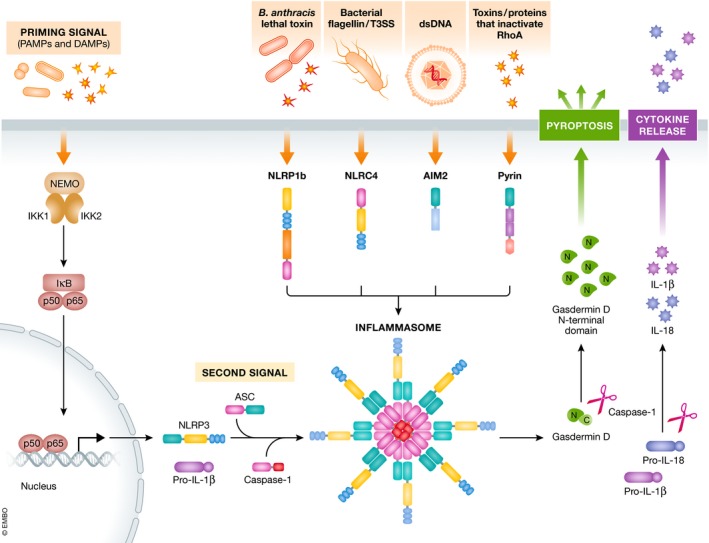
Inflammasomes assemble in a stimulus‐specific manner. Different DAMPs and PAMPs are able to induce NLRP3, while NLRP1b responds to Bacillus anthracis lethal toxin, NLRC4 recognizes bacterial flagellin and/or the type III secretion system of bacterial pathogens, AIM2 is specifically activated by dsDNA, and pyrin recognizes the inactivation of RhoA by toxins and effector proteins. Activation of the NLRP3 inflammasome involves a two‐step mechanism. The priming signal is detected by membrane‐bound PRRs, including TLRs and C‐type lectin receptors (CLRs) and induces NF‐κB‐dependent transcription of NLRP3 and pro‐IL‐1β precursor protein, and controls post‐translational modifications that license NLRP3 activation. The second activation signal is necessary for inflammasome formation, depending on the oligomerization and subsequent activation of procaspase‐1. Active caspase‐1 then cleaves pro‐IL‐1β and pro‐IL‐18 to their mature forms IL‐1β and IL‐18 which get secreted. In addition, caspase‐1 can cleave gasdermin D, releasing its N‐terminal fragment which translocates to the plasma membrane inducing pore formation and pyroptotic cell death. In contrast to NLRP3, other inflammasome receptors do not need this initial priming signal to induce inflammasome activation and cytokine release.
