Figure 1.
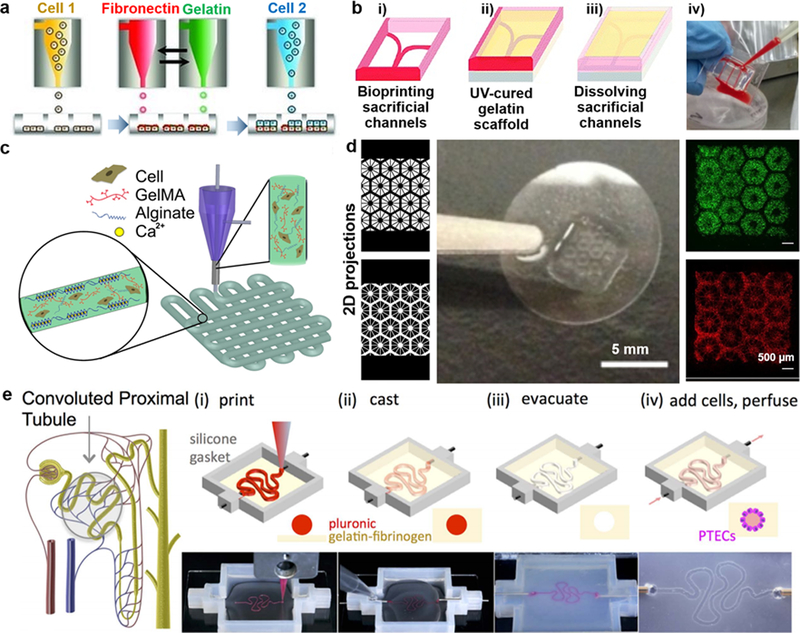
Representative OoC models: a) Schematic of the development of liver micro‐tissue (as spheroids) by inkjet bioprinting of endothelial cells (cell 1), a thin layer of fibronectin and gelatin, and hepatocytes (HPCs; cell 2); reprinted with permission from Matsusaki et al. 2013.2 b) Schematic of the blood-vessel-on-a-chip using bioprinting process of sacrificial channel (i), adding GelMA (gelatin methacryloyl) (ii) and removing channels (iii), along with the infusion of human whole blood into the endothelialized microchannels (iv); adapted with permission from Zhang et al. 2016.4 c) Schematic showing the extrusion of a mixture of alginate and GelMA for the heart-on-a-chip; reprinted with permission from Zhang et al. 2016.5 d) Liver model in which human induced pluripotent stem cells (hiPSCs)-HPCs were patterned by the first digital mask (or two-dimensional projection) followed by the patterning of supporting cells using a second digital mask, shown with fluorescent images showing patterns of fluorescently labeled hiPSC-HPCs (green) and supporting cells (red), along with a photograph of the final chip (center); adapted with permission from Ma et al. 2016.8 e) Schematic of a nephron highlighting the convoluted proximal tubule, along with corresponding schematic and images of different steps in the fabrication of proximal tubules: a sacrificial ink is first printed on a gelatin-fibrinogen extracellular matrix (i), additional matrix is cast around the printed feature (ii), the sacrificial ink is evacuated to create an open tubule (iii), and Proximal tubule epithelial cells (PTECs) are seeded within the tubule and perfused for long time periods (iv); reprinted with permission from Homan et al. 2016.3
