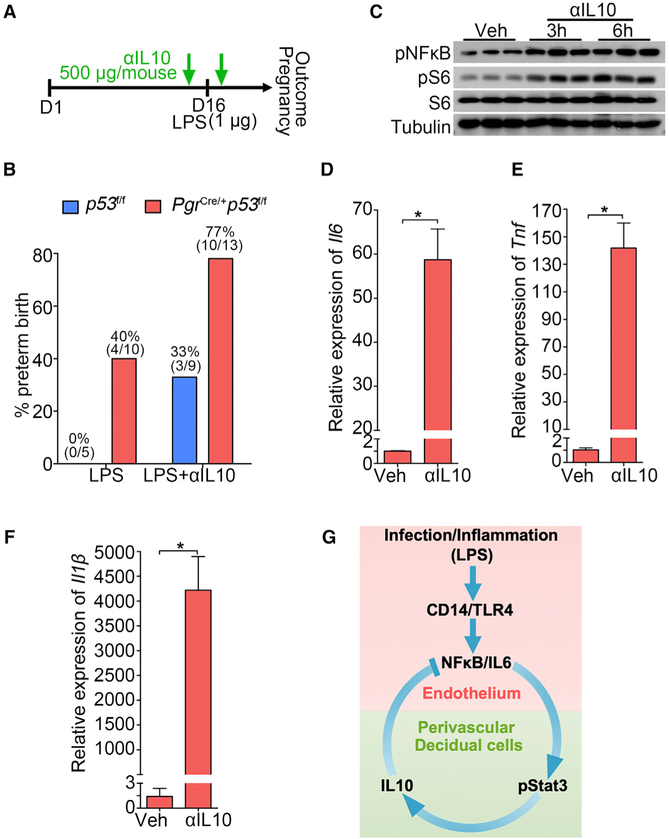
Figure 7. The Role of IL-10 in PTB Prevention.
(A) A scheme of treatment schedule of IL-10 neutralizing antibody (αIL-10).
(B) Preterm birth rate of p53f/f and PgrCre/+p53f/f mice after IL-10 neutralizing antibody (αIL-10) injection in the presence of a ultra-low dose of LPS exposure (1 μg/mouse). The number in parentheses indicates the incidence of PTB in total number of mice analyzed.
(C) Western blot results for pNFkB and pS6 levels in decidualized stromal cells after IL-10 neutralizing antibody treatment for 3 and 6 hours.
(D–F) Il6 (D), Il1b (E), and Tnf (F) mRNA levels by IL-10 neutralizing antibody treatment. n = 3, *p < 0.05. Data are represented as mean ± SEM (n = 3).
(G) A representative scheme of a physiological role of endothelial TLR4 in LPS-induced inflammation. After binding with LPS, endothelial TLR4 directs NFkB nuclear translocation to stimulate endothelial IL-6 expression in endothelial cells. Locally generated IL-6 stimulates Stat3 phosphorylation in perivascular decidual cells for IL-10 expression. Activation of endothelial TLR4 by LPS, which counters endogenous IL-10 production, results in preterm birth. CD14 is a co-receptor for TLR4 and highly induced in endothelial cells after LPS stimulation.