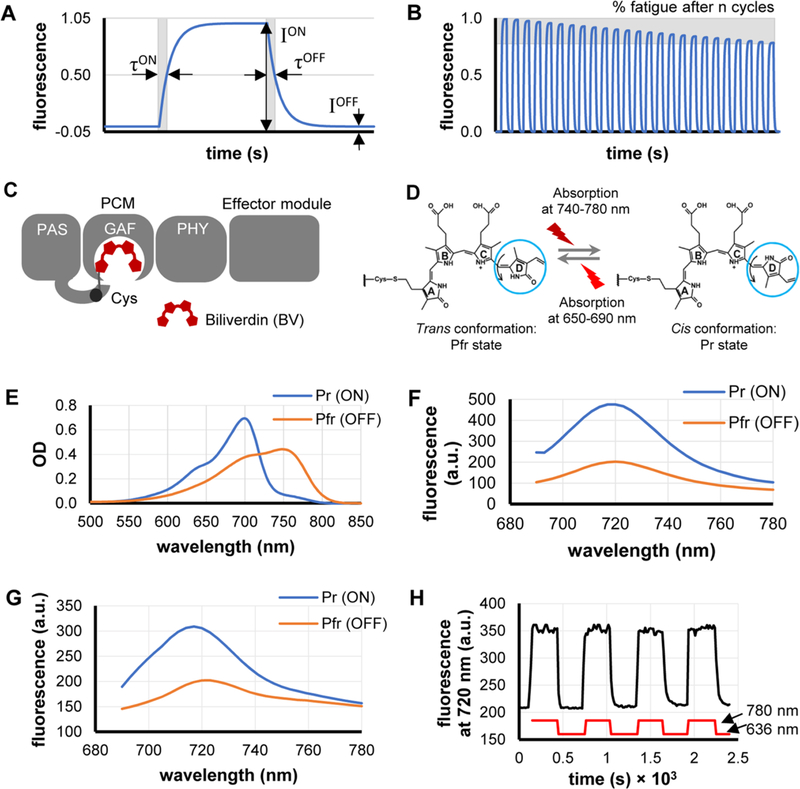
Figure 1.
Characterization of DrBphP-PCM reversible photoswitching and major parameters that should be optimized. (A) Each rsFP photoswitching cycle is characterized by switching contrast, which is the ratio of fluorescence intensities (ION/IOFF), as well as by half-times of photoswitching ON (τON) and OFF (τOFF). (B) Photofatigue of an rsFP is denoted as the relative decrease of the ION fluorescence intensity after n cycles of reversible switching. (C) A monomer of the full-length DrBphP bacterial phytochrome consists of a PCM module (PAS, GAF, and PHY domains) and an effector module. (D) Biliverdin (BV) molecule bounded to DrBphP undergoes a cis–trans isomerization of the C15/C16 double bond in its D-ring (blue circle) during photoconversion. (E, F) Absorbance (panel (E)) and fluorescence (panel (F)) spectra of DrBphP-PCM protein solution in the Pr state (ON; blue line) and after photoconversion to the Pfr state (OFF; orange line). (G) Fluorescence spectra of the suspension of live HeLa S3 cells stably expressing DrBphP-PCM protein in the Pr state (ON; blue line) and after photoconversion to the Pfr state (OFF; orange line). (H) Repeated fluorescence changes of the suspension of HeLa S3 cells stably expressing DrBphP-PCM protein detected at 720 nm during recurrent illumination cycles with 780/20 nm (switching on) and 636/20 nm (switching off). Measurements in panels (E)–(H) were performed in cuvettes in a commercial spectrophotometer (panel (E)) and steady-state fluorometer (panels (F)–(H)).