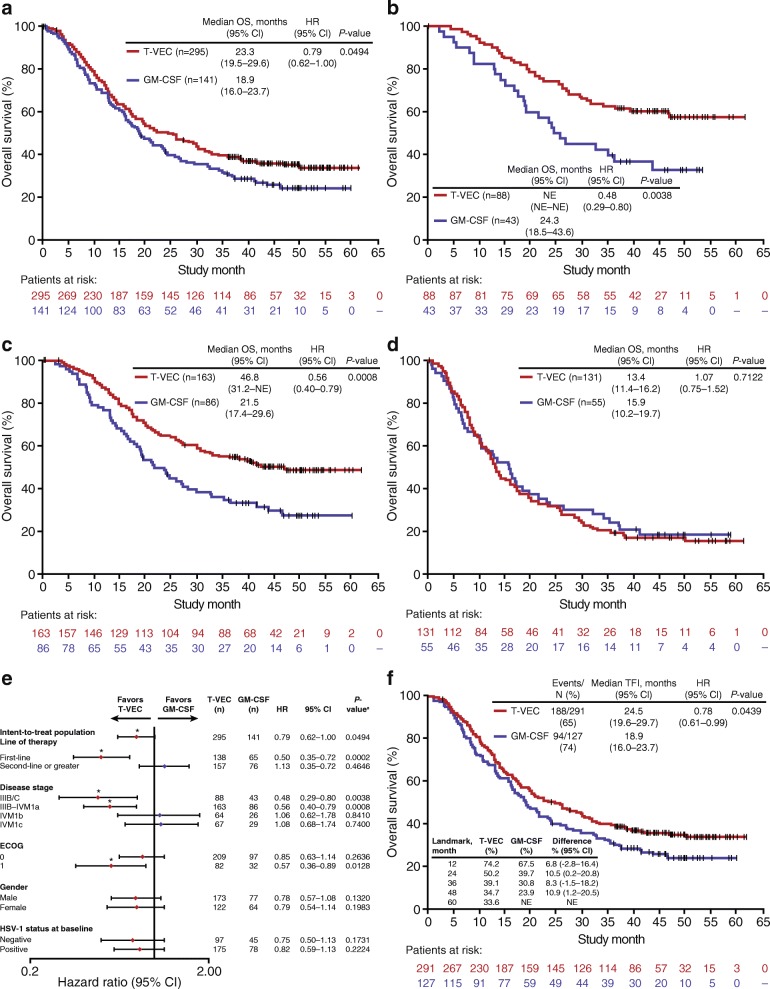
Fig. 3.
Final planned analyses of OS. a Final OS in the intent-to-treat population; b Final OS in patients with stage IIIB/C melanoma; c Final OS in patients with stage IIIB-IVM1a melanoma; d Final OS in patients with stage IVM1b/c melanoma; e Exploratory subgroup analyses of final OS (intent-to-treat population); and f Final OS in the treatment-received population (all randomized patients excluding those who did not receive allocated treatment; n = 4 in the talimogene laherparepvec arm; n = 14 in the GM-CSF arm). aP-values are descriptive and represent the statistical significance of the treatment difference within the subgroup from log-rank test unless otherwise stated (*P < 0.05). CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GM-CSF, granulocyte-macrophage colony-stimulating factor; HR, hazard ratio; NE, not estimable; OS, overall survival; T-VEC, talimogene laherparepvec