Abstract
Breast cancer remains the most commonly diagnosed cancer in Chinese women. Paclitaxel (PTX) is a chemotherapy medication used to treat breast cancer patients. However, a side effect of paclitaxel is the severe drug resistance. Previous studies demonstrated that dysregulation of microRNAs could regulate sensitivity to paclitaxel in breast cancer. Here, the present study aimed to lucubrate the underlying mechanisms of miR-107 in regulating the sensitivity of breast cancer cells to PTX. The results demonstrated that miR-107 was down-regulated in breast cancer tumor tissues, while TPD52 was significantly up-regulated compared with the non-tumor adjacent tissues. After confirming that TPD52 may be a major target of miR-107 via a dual-luciferase reporter assay, the western blot and RT-qPCR assays further demonstrated that miR-107 may reduce the expression level of TPD52 as well. In addition, miR-107 may prominently enhance PTX induced reduction of cell viability and the promotion of cell apoptosis in breast cancer, and the variation could be reversed by co-transfected with pcDNA3.1-TPD52. Finally, miR-107 could further reduce the decreased expression of TPD52, Wnt1, β-catenin and cyclin D1 that was induced by PTX in both mRNA and protein levels, which were rescued by pcDNA3.1-TPD52 indicating that miR-107 regulated breast cancer cell sensitivity to PTX may be targeting TPD52 through Wnt/β-catenin signaling pathway.
Keywords: miR-107, TPD52, Breast cancer, Paclitaxel
1. Introduction
Breast cancer is now the most frequently diagnosed cancer [1], remaining the sixth leading cause of cancer-related death in Chinese women [2]. Besides surgery, chemotherapy such as paclitaxel (PTX) is used for cases of breast cancer in stage 2-4 [3]. PTX is a chemotherapy medication used to treat various cancers, including breast cancer [4]. However, one of the serious side effects which could interfere with breast cancer treatment efficiency is drug resistance [5]. Therefore, it’s urging to increase the sensitivity of breast cancer cells to PTX in order to improve clinical treatment efficiency.
microRNAs (miRNAs) are a group of long small non-coding RNAs with ~22 nucleotides [6], involving in the regulation of many cellular processes, such as cell proliferation [7], apoptosis [8] and differentiation [9]. Furthermore, microRNAs were found to be dysregulated in various cancers, including breast cancer [10]. A previous study reported that miR-107 could inhibit proliferation and migration in breast cancer [11]. That is to say, miR-107 may function as a tumor suppressor candidate in breast cancer. In addition to regulating tumor evolution and development, microRNAs were also confirmed to participate in regulating the sensitivity of breast cancer cells to PTX [12]. However, whether or not miR-107 could regulate sensitivity to PTX in breast cancer still remains to be lucubrated.
Hence, in the present study, we aimed to demonstrate the underlying mechanisms of miR-107 on sensitivity to PTX in breast cancer. Based on the results, miR-107 may induce sensitivity of breast cancer cells to PTX may be targeting TPD52 through Wnt/β-catenin signaling pathway.
2. Materials and methods
2.1. Tissues
A total of 35 human breast cancer tumor tissues and non-tumor adjacent tissues were obtained from Tianjin Baodi People’s Hospital between June 2014 and October 2016 with the informed consent of patients pathologically diagnosed with breast cancer (Table 1). All the patients underwent surgical resection without receiving immunotherapy, chemotherapy or radiotherapy. Tissues were preserved in liquid nitrogen and stored at -80°C. This study obtained the approval of the appropriated ethics committee from Tianjin Baodi People’s Hospital.
Table 1.
Clinicopathological features in 35 breast cancer tumor specimens
| Clinicopathological variable | Cases | Expression level miR-107 high (n=20) | miR-107 low (n=15) | P value (*P<0.05) |
|---|---|---|---|---|
| Age | ||||
| ≤60 years | 6 | 2 | 4 | 0.605 |
| >60 years | 29 | 13 | 16 | |
| Tumor size | ||||
| ≥2cm | 23 | 6 | 17 | 0.006 |
| <2cm | 12 | 9 | 3 | |
| Fertility | ||||
| Yes | 23 | 14 | 9 | |
| No | 12 | 1 | 11 | 0.003 |
| Drinking | ||||
| Yes | 12 | 2 | 10 | |
| No | 23 | 13 | 10 | 0.023 |
| TNM stage | ||||
| I/II | 14 | 9 | 5 | 0.036 |
| III/IV | 21 | 6 | 15 |
2.2. Cell culture
Human breast cancer cell line MCF-7 was purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MCF-7 cell line was cultivated at 37°C under 5% CO2 in Roswell Park Memorial Institute (RPMI; Gibco®, Thermo Fisher Scientific, Inc., Waltham, MA, USA) 1640 medium with 10% fetal bovine serum (MP Biomedicals, Costa Mesa, CA, USA) containing penicillin-streptomycin (Sigma-Aldrich, Shanghai, China).
2.3. Dual-luciferase reporter assay
The MCF-10A cells (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were seeded in 24-well plates with the density of 1×105 cells/well. Meanwhile, TPD52 3’UTR was amplified from cDNA of MCF-10A cells and inserted into pGL-3-Basic (Fenghbio, Changsha, China). Then, the MCF-7 cells were co-transfected with either wild-type or mutant TPD52 3’UTR, in combination with miR-NC mimics or miR-107 mimics via Lipofectamine® 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA). After transfection, the cells were cultivated at 37°C, 5 % CO2 for 48 h. Afterward, cells were harvested and the luciferase activities were quantified using a dual-luciferase assay kit (Promega, Madison, WI, USA) following the manufacturer’s protocol. All the procedures were repeated in triplicate.
2.4. Cell transfection
The miR-NC mimics and miR-107 mimics were obtained from GenePharma (Shanghai, China). In order to confirm the transfection efficiency of miR-107, cells were divided into three different groups: i) the control group, un-treated cells; ii) miR-NC mimics group, cells transfected with miR-NC mimics and iii) miR-107 mimics group, cells transfected with miR-107 mimics. The MCF-7 cell line was seeded into 6-well plate with the density of 3×105 cells/well, then the miR-NC mimics or miR-107 mimics were transfected into cells with Lipofectamine® 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions.
2.5. Vector reconstruction and transfection
A TPD52-expressing vector was constructed by inserting the TPD52 expression into the pcDNA3.1 vector. In brief, full-length TPD52 cDNA was cloned from MCF-10A cells
and reconstructed into the pcDNA3.1 vector (GeneChem, Shanghai, China). In order to confirm the transfection efficiency of TPD52, cells were divided into three different groups: i) the control group, un-treated cells; ii) pcDNA3.1 group, cells transfected with pcDNA3.1 and iii) pcD-NA3.1-TPD52, cells transfected with pcDNA3.1-TPD52. The MCF-7 cells were seeded into 6-well plate with the density of 3×105 cells/well, then the pcDNA3.1 or pcDNA3.1-TPD52 were transfected with Lipofectamine® 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions.
2.6. The cell viability confirmed by MTT assay
According to the study, the following experiments were divided into five different groups: i) the control group, un-treated cells; ii) the PTX group, cells were exposed to PTX with the concentration of 0.1 μg/ml before transfection; iii) PTX + NC group, cells were exposed to PTX and then transfected with miR-107 NC mimics; iv) PTX + miR-107 group, cells were exposed to PTX and then transfected with miR-107 mimics; and v) PTX + TPD52 + miR-107, cells were exposed to PTX and then transfected with miR-107 mimics and pcDNA3.1-TPD52.
Cell viability was determined using the MTT kit (3-(4.5-Dimethylghiazol-2-yl)-2,5-diphenyltetrazolium Bromide; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Briefly, cells were seeded on a 96-well plate with the density of 5×103 cells/well and cultivated for 24 h at 37°C and 5% CO2. Refer to a previous study [13], MCF-7 cells were exposed to PTX with the concentration of 0.1 μg/ml for 2 h (PTX; Sigma-Aldrich, St. Louis, MO, US) before transfection. Subsequently, 20 μl 5 mg/ml MTT was added into each well and culture for 4 h, and 100 μl DMSO (dimethyl sulfoxide; Solarbio®, Shanghai, China) was added in each well of the plate in order to dissolve the formazan crystal formed. Finally, the absorbance was read at 490 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The relative cell viability in different groups was quantified by comparing the absorbance values of treated cells with the un-treated cells. All the procedures were repeated in triplicate.
2.7. The apoptosis rate of breast cancer cells confirmed by flow cytometry assay
MCF-7 cells were seeded on a 6-well plate with a density of 3 × 105 cells/well and then exposed to PTX (with the concentration of 0.1 μg/ml) for 2 h. Then, cells were transfected and cultivated at 37°C and 5% CO2 for 48 h. After digested with 0.25% trypsin (Sigma-Aldrich, Shanghai, China) and washed with phosphate-buffered saline (PBS; Solarbio®, Beijing, China), cells were stained with Annexin V-FITC/PI reagent kit (Sigma-Aldrich, Shanghai, China) in the dark for 10 min. Cell apoptosis rate was evaluated via a flow cytometry (Beckman Coulter, Inc., CA, USA) and CellQuest Pro v.5.2 software (BD FACScan; BD Biosciences, Franklin Lakes, NJ, USA). All the procedures were repeated in triplicate.
RNA extraction and RT-qPCR assay. Total RNA was extracted from cells or tissues using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions. Meanwhile, miRNA was isolated from cells or tissues using the miR-Neasy Mini Kit (Qiagen, Valencia, CA, USA). Afterward, TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) was applied to synthesize cDNA from miRNA. Meanwhile, 2 μl RNA was reverse transcribed into cDNA using RrimeScript RT reagent kit (Epicentre, Madison, WI, USA) followed by RT-qPCR (Real-time Quantitative polymerase chain reaction) analysis. Briefly, the PCR reaction conditions were described as followed: i) denatured for 3 min at 95°C; ii) followed by the thermal cycling parameters with 35 cycles of denaturation for 30 s at 95°C, annealing for 45 s at 62°C, extension for 30 s at 72°C; and iii) extension for 10 min at 72°C. The relative expression level of miR-107 was normalized to U6, while the expression levels of TPD52 and related proteins were normalized to GAPDH according to the 2-△△Ct method. All the procedures were repeated in triplicate. The primer sequences were described as followed: miR-107 forward, 5’-GCCGAATTCAAAGCGAGATTCCATCAGCA-3’ and reverse, 5’-GCCGGATCCTGTCAACCCAGAACTCAAAGG-3’; U6 forward, 5’-CTCGCTTCGGCAGCACA-3’ and reverse, 5’-AACGCTTCACGAATTTGCGT-3’; TPD52 forward, 5’-AACAGAACATTGCCAAAGGGTG-3’ and reverse, 5’-TGACTGAGCCAACAGACGAAA-3’; Wnt1 forward, 5’-ATAGCCTCCTCCACGAACCT-3’ and reverse, 5’-GGAATTGCCACTTGCACTCT-3’; β-catenin forward, 5’-GCTGATTTGATGGAGTTGGA-3’ and reverse, 5’-TCAGCTACTTGTTCTTGAGTGAA-3’; Cyclin D1 forward, 5’-GGATGCTGGAGGTCTGCGAG-3’ and reverse, 5’-GAGAGGAAGCGTGTGAGGCG-3’; GAPDH forward, 5’-TGTTCGTCATGGGTGTGAAC-3’ and reverse, 5’-ATGGCATGGACTGTGGTCAT-3’.
2.8. Western blot
After 48 h transfection, cells in different groups were harvested and lysed by the RIPA buffer (Solarbio®, Beijing, China) and a protease inhibitor cocktail (Apexbio, Shanghai, China). And the concentration of the protein was quantified using a Bradford Protein Quantification Kit (Yeasen, Shanghai, China). Then, equal amounts (20 μg) of the cell lysates were separated by 10% SDS-PAGE (Bio-Rad, Hercule, CA, USA) and transferred onto polyvinylidene difluoride membranes (PVDF; Roche, Switzerland). Membranes were then blocked with 5% non-fat milk buffer at 37°C for 50 min. Afterward, the primary antibodies were added on the membranes at 37°C overnight: rabbit anti-TPD52 (1:1000; ab182578; Abcam, Cambridge, MA, USA); rabbit anti-Wnt1 (1:1000; ab15251; Abcam, Cambridge, MA, USA); anti-β-catenin (1:5000; ab32572; Abcam, Cambridge, MA, USA); rabbit anti-Cyclin D1 (1:10000; ab134175; Abcam, Cambridge, MA, USA) and rabbit anti-GAPDH (1:2500; ab9485; Abcam, Cambridge, MA, USA). After washing with phosphate-buffered saline (PBS; Solarbio®, Beijing, China) containing 0.1% Tween 20 (Sigma-Aldrich, Shanghai, China), the secondary antibody IgG H&L (HRP; 1:1000; ab7090; Abcam, Cambridge, MA, USA) was added at 37°C for 2 h. Finally, the membranes were washed with phosphate-buffered saline (PBS; Solarbio®, Beijing, China) containing 0.1% Tween 20 (Sigma-Aldrich, Shanghai, China). After that, the enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA) was added to the membrane. Acquisition and Analysis Software (UVP, Upland, CA, USA) was applied to determine the gray values of proteins. GAPDH functioned as the internal control. All the procedures were repeated in triplicate.
2.9. Statistical analysis
The SPSS version 18.0 statistical software ( SPSS; Chicago, IL, USA) was applied to analyze the data. Data were presented as the means ± standard deviation (SD). In table 1, the comparison of patients characteristics was achieved using the χ2 test. P<0.05 was considered to be statistically significant. Differences between two groups were assessed by student’s t-test method, while the one-way analysis of variation (ANOVA) followed by Newman-Keuls analysis was used to distinguish differences among the three groups or more.
3. Results
3.1. The expression level of miR-107 was down-regulated in breast cancer tumor tissues
As demonstrated in Fig. 1, the expression level of miR-107 was significantly decreased in the breast cancer tumor tissues, compared with non-tumor adjacent tissues (P<0.01).
Figure 1.
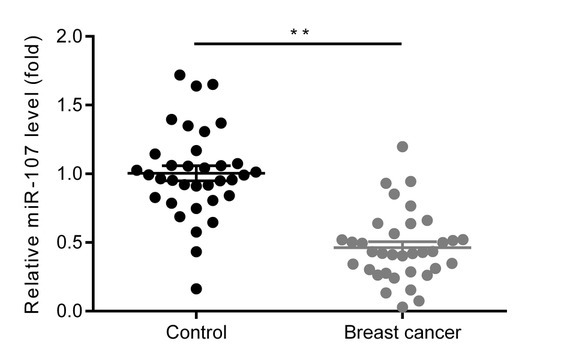
The expression level of miR-107 is down-regulated in breast cancer tumor tissues. The relative expression level of miR-107 in breast cancer tumor tissues and non-tumor adjacent tissues were compared via RT-qPCR assay. **P<0.01, vs control group. Control, non-tumor adjacent tissues; breast cancer, breast cancer tumor tissues; miR-107, microRNA-107.
3.2. TPD52 may be a target of miR-107
As demonstrated in Fig. 2A, the 3’UTR of the gene TPD52 was found to contain the binding sequence at the position 34-31 for the miR-107, implying that TPTD52 could be a downstream target gene of miR-107. Moreover, Fig. 2B further demonstrated that the relative luciferase activities in the wild-type (WT) TPD52 3’UTR plasmid-transfected cells were remarkably reduced by the transfection of miR-107 mimics, however, there were no differences in the mutant-type TPD52 3’UTR (TPD52-3’UTR) plasmid-transfected cells (P<0.01).
Figure 2.
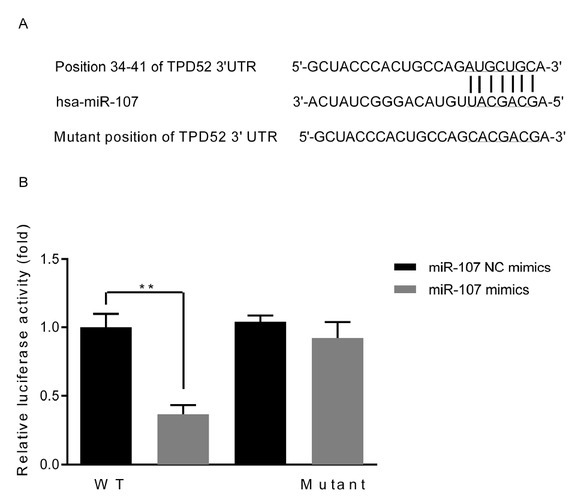
TPD52 is potentially a major target of miR-107. (A) Sequence alignment of the paired site of the 3’ URT of miR-107 and TPD52 ; (B) The luciferase activity in different groups. **P<0.01, vs miR-NC mimics group. WT, wild-type; TPD52, tumor protein D52; UTR, untranslated region; miR-107, microRNA-107; miR-107 NC mimics, microRNA-107 mimics negative control-transfected cells; miR-107 mimics, microRNA-107 mimics-transfected cells.
3.3. The expression level of TPD52 was up-regulated in breast cancer tumor tissues
As demonstrated in Fig. 3A, the expression level of TPD52 was significantly increased in the breast cancer tumor tissues, compared with non-tumor adjacent tissues via RT-qPCR assay (P<0.01). Meanwhile, the western blot
Figure 3.
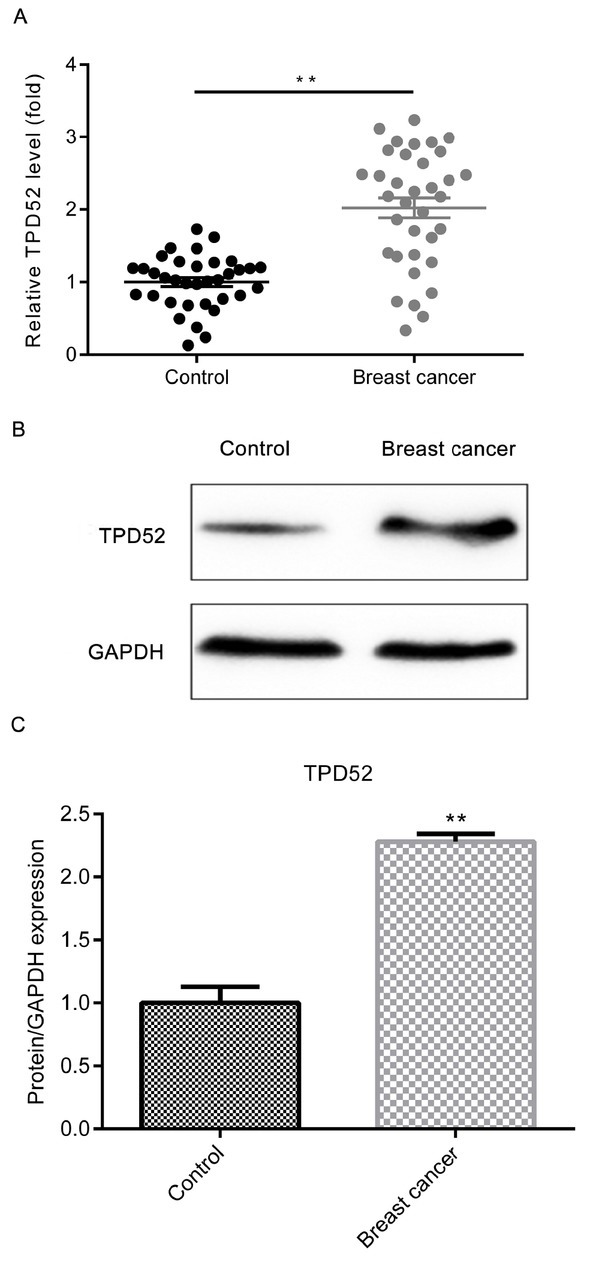
The expression level of TPD52 is up-regulated in breast cancer tumor tissues. (A) Relative TPD52 mRNA expression level in breast cancer tumor tissues and non-tumor adjacent tissues was compared by RT-qPCR assay. (B) Relative protein expression level of TPD52 in breast cancer tumor tissues and non-tumor adjacent tissues was compared by western blot assay. (C) The quantitative result of B was presented. **P<0.01, vs control group. Control, non-tumor adjacent tissues; breast cancer, breast cancer tumor tissues; TPD52, tumor protein D52; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
assay presented the similar trend of variation as well (Fig. 3B and 3C; P<0.01).
3.4. The transfection efficiency was confirmed
As demonstrated in Fig. 4A, the expression level of miR-107 was significantly increased in the miR-107 mimics group, compared with miR-NC mimics group (P<0.01). As demonstrated in Fig. 4B, the expression level of TPD52 mRNA was significantly increased in the pcDNA3.1-TPD52 group, compared with the pcDNA3.1 group (P<0.01).
Figure 4.
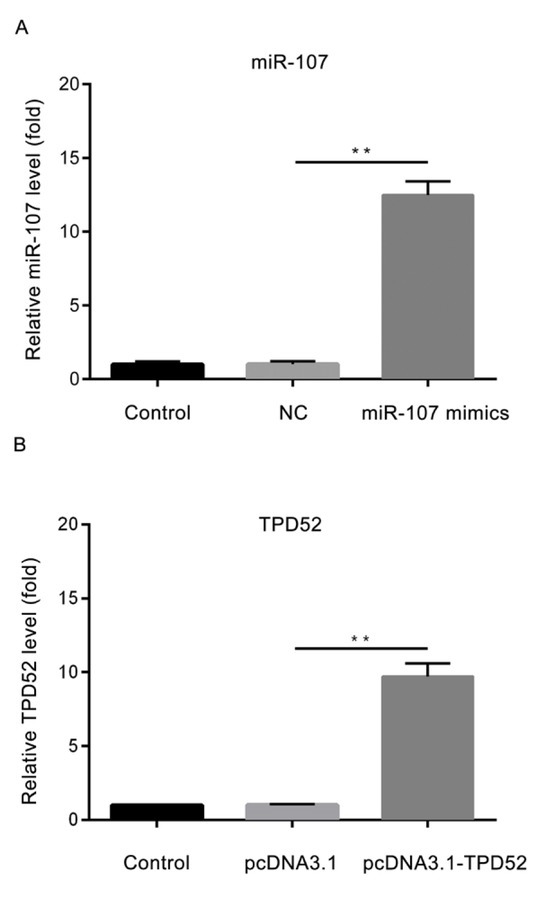
The transfection efficiency of miR-107 and TPD52. (A) The expression level of miR-107 in different groups; (B) The expression level of TPD52 in different groups. **P<0.01, miR-107 mimics vs NC; pcDNA3.1-TPD52 vs pcDNA3.1. Control, un-treated cells; NC, microRNA-107 mimics negative control-transfected cells; miR-107 mimics, microRNA-107 mimics-transfected cells; pcDNA3.1, cells transfected with pcDNA3.1; pcDNA3.1-TPD52, cells transfected with pcDNA3.1-TPD52; miR-107, microRNA-107; TPD52, tumor protein D52.
The expression level of miR-107 is significantly higher in miR-107 mimics group. The transfection efficiency of miR-107 was confirmed by RT-qPCR assay. **P<0.01, vs miR-NC mimics group. Control, untreated cells; NC, microRNA-107 mimics negative control-transfected cells; miR-107 mimics, microRNA-107 mimics-transfected cells.
3.5. miR-107 significantly reduced the expression level of TPD52
As demonstrated in Fig. 5A, the mRNA expression level of TDP52 was significantly decreased in the miR-107 mimics groups, in contrast with NC group (P<0.01). Meanwhile, the protein results in Fig. 5B and 5C presented the similar trend of variation as well (P<0.01).
Figure 5.
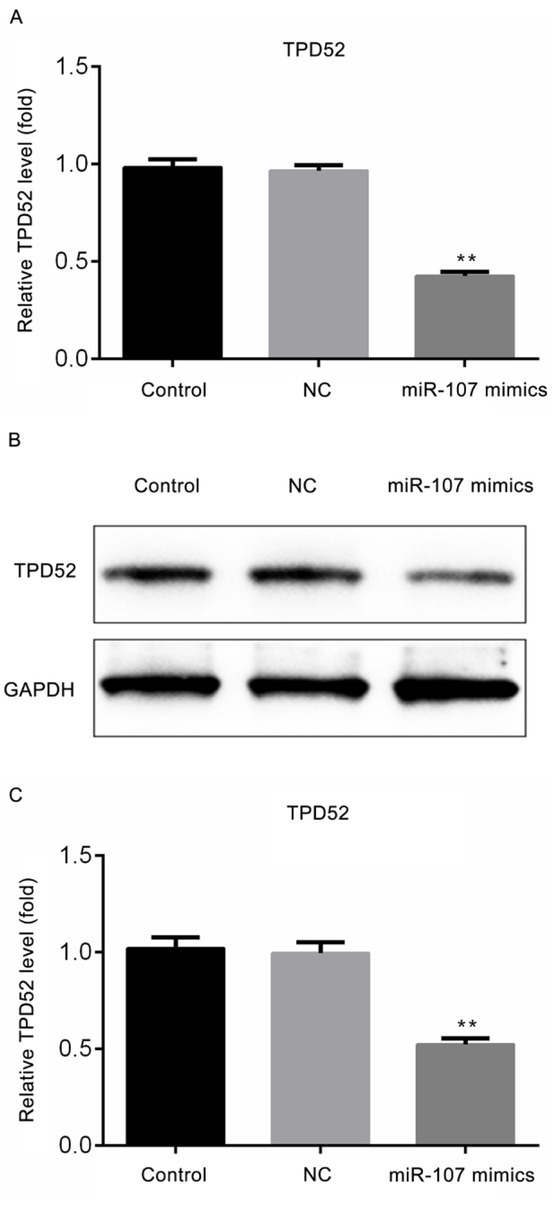
miR-107 reduces the expression level of TPD52. (A) The mRNA expression level of TPD52 in differing groups; (B) The protein expression level of TPD52 in different groups; (C) The quantitative result of B was presented. **P<0.01, vs NC group. Control, untreated cells; NC, microRNA-107 mimics negative control-transfected cells; miR-107 mimics, microRNA-107 mimics-transfected cells; TPD52, tumor protein D52; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
3.6. miR-107 significantly decreased breast cancer cell viability after treated with PTX
As to further illustrate the effect of miR-107 on the sensitivity of breast cancer cells to PTX, the cell viability was measured via MTT assay. As demonstrated in Fig. 6, the cell viability in the PTX group was markedly decreased compared to the control group (P<0.01), meanwhile, there were no differences in the PTX group and PTX + NC group. In contrast with PTX + NC group, the cell viability
Figure 6.
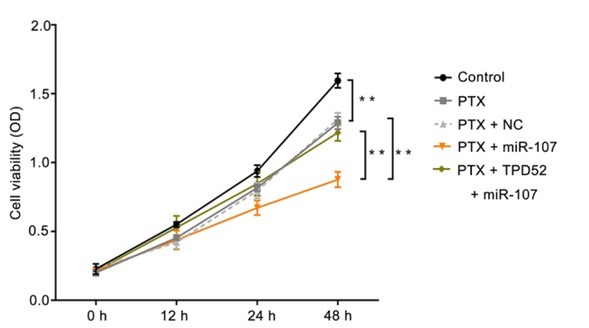
miR-107 significantly decreases breast cancer cell viability after treated with PTX. The cell viability of breast cancer cells in different groups was measured by the MTT assay. **P<0.01, PTX vs control; PTX + miR-107 vs PTX + NC; PTX + TPD52 + miR-107 vs PTX +miR-107. Control, un-treated cells; PTX, cells were exposed to PTX with the concentration of 0.1μg/ml; NC, miR-107 mimics negative control-transfected cells; miR-107, miR-107 mimics-transfected cells; TPD52, tumor protein D52, cells transfected with pcDNA3.1-TPD52.
in PTX + miR-107 was significantly decreased, while the variation could be reversed by co-transfected with the pcDNA3.1-TPD52 (P<0.01). Collectively, miR-107 may significantly reduce breast cancer cell viability after treated with PTX.
3.7. miR-107 significantly promoted breast cancer cell apoptosis rate after treated with PTX
As demonstrated in Fig. 7A-7F, compared with the control group, the apoptosis rate was remarkably higher after treated with PTX (P<0.01), while there was almost no variation between the PTX group and PTX + NC group. Moreover, after transfection, the apoptosis rate in miR-107 mimics group was remarkably higher than that in the PTX + miR-107 group (P<0.01), however, the variation could be reversed by co-transfected with the pcDNA3.1-TPD52 (P<0.01). Taken all together, miR-107 could significantly promote breast cancer cell apoptosis after treated with PTX.
Figure 7.
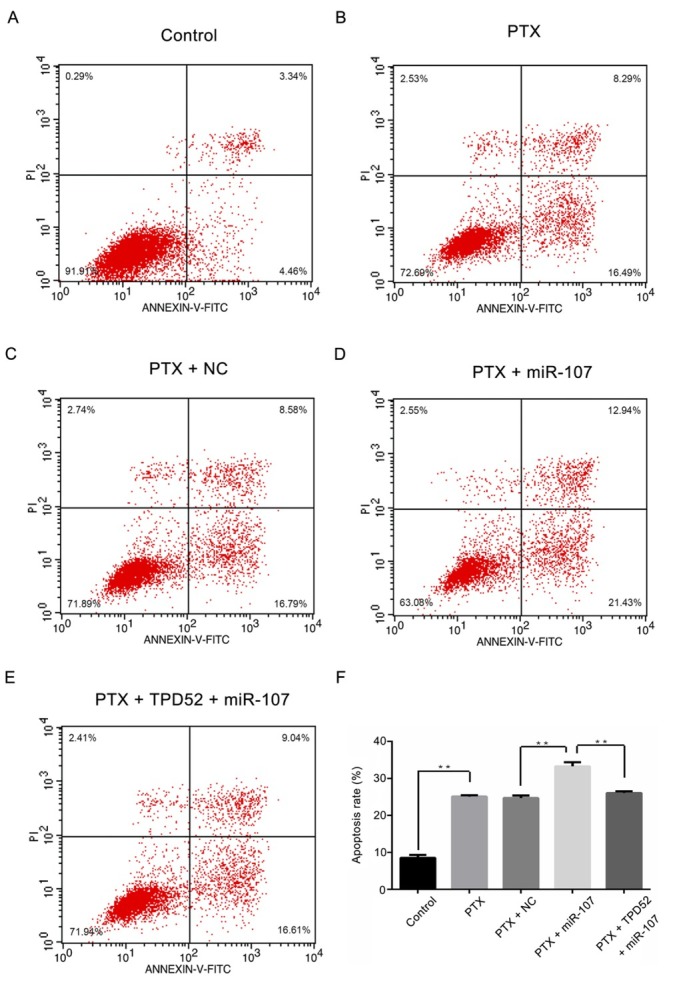
miR-107 significantly promotes breast cancer cell apoptosis rate after treated with paclitaxel. (A) Control group; (B) PTX group; (C) PTX + NC group; (D) PTX + miR-107 group; (E) PTX +TPD52 +miR-107 group; (F) The corresponding quantified apoptosis rate results of A-E was exhibited. **P<0.01, PTX vs control; PTX + miR-107 vs PTX + NC; PTX + TPD52 + miR-107 vs PTX +miR-107. Control, un-treated cells; PTX, cells were exposed to PTX with the concentration of 0.1μg/ml; NC, miR-107 mimics negative control-transfected cells; miR-107, miR-107 mimics-transfected cells; TPD52, tumor protein D52, cells transfected with pcDNA3.1-TPD52.
3.8. Effect of miR-107 on the expression levels of TPD52, Wnt1, β-catenin and Cyclin D1
As to further confirm whether and how miR-107 and TPD52 participated in the regulation of breast cancer cell viability and apoptosis, the relative expression levels of Wnt1, β-catenin and Cyclin D1 were measured by RT-qPCR and western blot assays. As demonstrated in Fig. 8A-8D, the mRNA expression levels of TPD52, Wnt1, β-catenin and cyclin D1 were significantly decreased after treated with PTX (**P<0.01; *P<0.05), while there were no differences between PTX group and PTX + NC group. Subsequently, after transfection, the expression levels of TPD52, Wnt1, β-catenin and Cyclin D1 in PTX + miR-107 were remarkably lower than those in the PTX + NC group (**P<0.01; *P<0.05), however, the variation could be reversed by co-transfected with pcDNA3.1-TPD52 (**P<0.01; *P<0.05). Meanwhile, consistent with the results of the RT-qPCR assay, the western blot data (Fig. 9A and 9B) presented the similar trend of variation (P<0.01).
Figure 8.
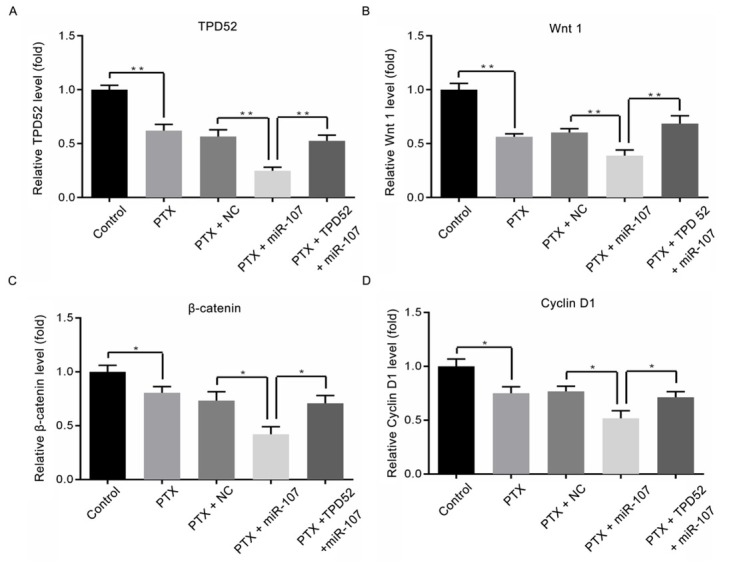
Effect of miR-107 on the mRNA expression levels of TPD52, Wnt1, β-catenin and cyclin D1. (A) The mRNA expression level of TPD52 in different groups; (B) The mRNA expression level of Wnt 1 in different groups; (C) The mRNA expression level of β-catenin in different groups; (D) The mRNA expression level of Cyclin D1 in different groups. *P<0.05, **P<0.01, PTX vs control; PTX + miR-107 vs PTX + NC; PTX + TPD52 + miR-107 vs PTX +miR-107. Control, un-treated cells; PTX, cells were exposed to PTX with the concentration of 0.1μg/ml; NC, miR-107 mimics negative control-transfected cells; miR-107, miR-107 mimics-transfected cells; TPD52, tumor protein D52, cells transfected with pcDNA3.1-TPD52.
Figure 9.
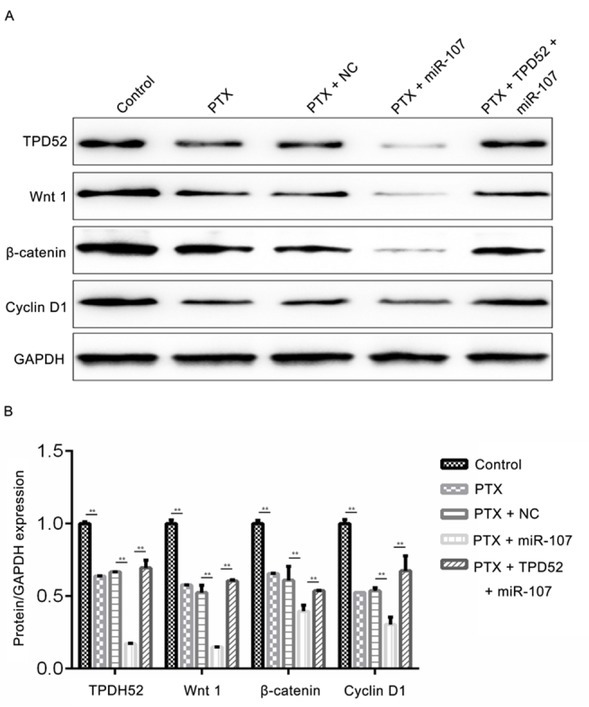
Effect of miR-107 on the protein expression levels of TPD52, Wnt1, β-catenin and cyclin D1. (A) The protein expression levels of TPD52, Wnt1, β-catenin and cyclin D1 in different groups; (B) The corresponding quantified results of A was presented. **P<0.01, PTX vs control; PTX + miR-107 vs PTX + NC; PTX + TPD52 + miR-107 vs PTX +miR-107. Control, un-treated cells; PTX, cells were exposed to PTX with the concentration of 0.1μg/ml; NC, miR-107 mimics negative control-transfected cells; miR-107, miR-107 mimics-transfected cells; TPD52, tumor protein D52, cells transfected with pcDNA3.1-TPD52; Wnt1, proto-oncogene protein wnt-1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
4. Discussion
In the present study, the underlying mechanisms of how miR-107 regulates the sensitivity of breast cancer cells to PTX were demonstrated. Firstly, the expression level of miR-107 was significantly lower in breast cancer tumor tissues. After confirming that TPD52 may be a target gene of miR-107, the expression level of TPD52 in breast cancer tumor tissues was further proved to be up-regulated, which were contrary to that of miR-107. Subsequently, the MTT and flow cytometry assays further illustrated that through targeting TPD52, miR-107 could significantly decrease breast cancer cell viability but promote apoptosis after treated with 0.1 μg/ml PTX. Finally, the western blot assay confirmed that miR-107 regulated the sensitivity of breast cancer cells to PTX by down-regulating the expression level of TPD52 through Wnt/β-catenin signaling pathway.
According to previous studies, miR-107 was proved to participate in regulating tumor development and evolution processes. For instance, miR-107 may suppress cell proliferation or promote apoptosis in lung cancer [14], gastric cancer [15] and breast cancer [16]. Another report also demonstrated that miR-107 was markedly downregulated in both breast cancer cell lines and breast tumors [16], functioning as an anti-metastasis factor in breast cancer as well [17]. Consistent with previous studies, the results in our study presented that the expression level of miR-107 was significantly decreased in breast cancer tumor tissues. Furthermore, miR-107 was considered to enhance chemosensitivity to PTX in NSCLC [18] and gastric cancer [19]. However, whether or not miR-107 participated in modulating PTX sensitivity in breast cancer remain to be lucubrated.
As the results presented, TPD52 was considered to be a target gene of miR-107 via a dual-luciferase reporter assay. Recently, TPD52 was reported to promote cell proliferation or suppress apoptosis in uterine leiomyoma cells [20], prostate cancer [21], and glioma cell [22]. TPD52 was also reported to be up-regulated in the breast cancer cell and promoting breast cancer metastasis as well [23, 24, 25], indicating that TPD52 functioned as the oncogene
in breast cancer. Consistent with previous studies, our results demonstrated that TPD52 was up-regulated in breast cancer tumor tissues. Furthermore, according to the MTT and flow cytometry assays, overexpression of miR-107 could further inhibit cell viability but promote apoptosis in breast cancer after treated with PTX, indicating that up-regulated miR-107 could enhance PTX sensitivity may be targeting TPD52 in breast cancer.
In order to lucubrate the underlying mechanisms of how miR-107 mediated the sensitivity to PTX in breast cancer cells, the expression levels of TPD52 and related proteins were measured by RT-qPCR and western blot assays. Wnt signaling pathway was first identified for its role in carcinogenesis and demonstrated by mutations leading to various diseases, including breast cancer [26,27]. Wnt1 protein is a member of Wnt signaling pathway proteins and a previous study also demonstrated that knockdown of wnt1 may induce apoptosis of human cancer cells [28], including breast cancer cells [29], indicating that wnt1 functioned as a tumor promoter in breast cancer. Wnt/β-catenin signaling pathway was considered to be involved in regulating tumor development in various cancers, including breast cancer [30,31]. Meanwhile, accumulating evidence illustrated that inhibition of Wnt/β-catenin pathway could suppress breast cancer proliferation or promote apoptosis as well [32, 33, 34, 35]. Cyclin D1 is a protein that in humans encoded by the CCND1 gene, and CCND1 genetic locus is commonly amplified in many human tumors including breast cancer [36]. Moreover, cyclin D1 overexpression was found in more than 50% of human breast cancers [37, 38], and suppression of cyclin D1 could inhibit breast cancer cell proliferation but induce apoptosis as well [39]. Recently, a study pointed out that knockdown of TPD52 could significantly decrease the expression level of cyclin D1 concerning cell apoptosis process [40]. According to our results, overexpressed miR-107 could further reduce PTX induced decrease of the expression levels of TPD52, Wnt1, β-catenin and cyclinD1 may be
targeting TPD52. Consistent with previous studies, we proposed the conjecture that suppression of TPD52 by up-regulating miR-107 could inhibit or inactivate Wnt/β-catenin signaling pathway and the expression level cyclin D1.
According to previous studies, TPD52 could activate or regulate NF-κB, PI3K/AKT, Raf/MEK/ERK or JAK/STAT3 signaling pathways concerning modulating cell proliferation and apoptosis [41,42]. However, whether or not other signal pathways beside the Wnt/β-catenin could regulate TPD52 remain to be further detected. Meanwhile, the underlying relationship and interaction between miR-107 and TPD52 need to be further detected in the future.
To sum up, miR-107 could enhance PTX sensitivity in breast cancer cells may be targeting TPD52 through Wnt/β-catenin signaling pathway.
Footnotes
Conflict of interest
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].Ahmedin J.D., Freddie B., Melissa M.C., Jacques F.M.E., Elizabeth W., David F.. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Chen W.C., Zheng R.S., Peter D.B., Zhang S.W., Zeng H.M., Freddie B.. Cancer statistics in China. CA: A Cancer Journal for Clinicians. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- [3].Nabholtz J.M., Gelmon K., Bontenbal M., Spielmann M., Catimel G., Conte P.. Multicancer, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. Journal of Clinical Oncology. 1996;14:1858–1867. doi: 10.1200/JCO.1996.14.6.1858. [DOI] [PubMed] [Google Scholar]
- [4].Paradaens R., Biganzoli L., Bruning P., Klijn J.G., Gamucci T., Housto S.. Paclitaxel versus doxorubicin as first-time single-agent chemotherapy for metastatic breast cancer: a European organization for research and treatment of cancer randomized study with cross-over. Journal of Clinical Oncology. 2000;18:724–733. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- [5].Mechetner E., Kyshtoobayeva A., Zonis S., Kim H., Stroup R., Garcia R.. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clinical Cancer Research. 1998;4:389–398. [PubMed] [Google Scholar]
- [6].Ambros V.. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- [7].Liang B., Yin J.J., Zhan X.R.. MiR-301a promotes cell proliferation by directly targeting TIMP2 in multiple myeloma. International Journal of Clinical and Experimental Pathology. 2015;8:9168–9174. [PMC free article] [PubMed] [Google Scholar]
- [8].Guo J., Li M., Meng X., Sui J., Dou L., Tang W.. MiR-291b-3p induces apoptosis in liver cell line NCTC1469 by reducing the level of RNA-binding protein HuR. Cell Physiology and Biochemistry. 2014;33:810–822. doi: 10.1159/000358654. [DOI] [PubMed] [Google Scholar]
- [9].Antoniou A., Mastroyiannopoulos N.P., Uney J.B., Phylactou L.A.. miR-186 inhibits muscle cell differentiation through myogenin regulation. The Journal of Biological Chemistry. 2014;289:3923–3935. doi: 10.1074/jbc.M113.507343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li P., Sheng C., Huang L., Zhang H., Huang L., Cheng Z.. MiR-183/-96/-182 cluster is up-regulated in most breast cancers and increases cell proliferation and migration. Breast Cancer Research. 2014;16:473. doi: 10.1186/s13058-014-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li X.Y., Luo Q.F., Wei C.K., Li D.F., Li J., Fang L.. miRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer. International Journal of Clinical and Experimental Pathology. 2014;7:32–40. [PMC free article] [PubMed] [Google Scholar]
- [12].Liu X.P., Tang H.L., Chen J.P., Song C.L., Yang L., Liu P.. microRNA-101 inhibits cell progression and increases paclitaxel sensitivity by suppressing MCL-1 expression in human triple-negative breast cancer. Oncotarget. 2015;6:20070–20083. doi: 10.18632/oncotarget.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang B., Zhao R., He Y., Fu X., Fu L., Zhu Z.. Micro RNA 100 sensitizes luminal A breast cancer cells to paclitaxel treatment in part by targeting mTOR. Oncotarget. 2016;7:5702–5714. doi: 10.18632/oncotarget.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang P., Liu X., Shao Y., Wang H., Liang C., Han B.. microRNA-107-5p suppresses non-small cell lung cancer by directly targeting oncogene epidermal growth factor receptor. Oncotarget. 2017;8:57012–57023. doi: 10.18632/oncotarget.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Song Y.Q., Ma X.H., Ma G.L., Lin B., Liu C., Deng Q.J.. microRNA-107 promotes proliferation of gastric cancer cells by targeting cyclin-dependent kinase 8. BMC. 2014;9:164. doi: 10.1186/s13000-014-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao B., Hao S., Tian W.G., Jiang Y., Zhang S., Guo L.J.. microRNA-107 is downregulated and having tumor suppressive effect in breast cancer by negatively regulating brain-derived neurotrophic factor. Journal of Gene Medicine. 2016;19:e2932. doi: 10.1002/jgm.2932. [DOI] [PubMed] [Google Scholar]
- [17].Isabel S., Brigitte R., Wolfgang J., Bernadette J., Klaus P., Heidi S.. Aberrant plasma levels of circulating miR-16, miR-107, miR-130a and miR-146a are associated with lymph node metastasis and receptor of breast cancer patients. Oncotarget. 2015;6:13387–13401. doi: 10.18632/oncotarget.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu C.J., Xie Z.B., Peng Q.Z.. miRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. American Journal of Cancer Research. 2017;7:1863–1873. [PMC free article] [PubMed] [Google Scholar]
- [19].Teng R., Hu Y., Zhou J., Seifer B., Chen Y., Shen J.. Overexpression of Lin28 decreases the chemosensitivity of gastric cancer cells to oxaliplatin, paclitaxel, doxorubicin, and fluorouracil in part via microRNA-107. PLoS One. 2015;10:e0143716. doi: 10.1371/journal.pone.0143716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen H., Xu H., Meng Y.G., Zhang Y., Chen J.Y., Wei X.N.. miR-139-5p regulates proliferation, apoptosis, and cell cycle of uterine leiomyoma cells by targeting TPD52. OncoTargets and Therapy. 2016;9:6151–6160. doi: 10.2147/OTT.S108890. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [21].Han G.Y., Fan M.C., Zhang X.J.. microRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochemical and Biophysical Research Communications. 2015;456:804–809. doi: 10.1016/j.bbrc.2014.12.026. [DOI] [PubMed] [Google Scholar]
- [22].Zhang Y., Li Y., Wang J., Lei P.. Long non-coding RNA ferritin heavy polypeptide 1 pseudogene 3 controls glioma cell proliferation and apoptosis via regulation of the microRNA-334-5p/tumor protein D52 axis. Molecular Medicine Reports. 2018;14 doi: 10.3892/mmr.2018.9491. 10.3892\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li G.D., Yao L., Zhang J.N., Li X.L., Dang S.W., Zeng K.. Tumor-suppressive microRNA-34a inhibits breast cancer cell migration and invasion via targeting oncogenic TPD52. Tumor Biology. 2016;6:7481–7491. doi: 10.1007/s13277-015-4623-4. [DOI] [PubMed] [Google Scholar]
- [24].Tennstedt P., Bolch C., Strobel G., Minner S., Burkhardt L., Grob T.. Patterns of TPD52 overexpression in multiple human solid tumor types analyzed by quantitative PCR. International Journal of Oncology. 2014;44:609–615. doi: 10.3892/ijo.2013.2200. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Z.L., Wang J.W., Gao R.F., Yang X., Zhang Y.F., Li J.. Downregulation of microRNA-449 promotes migration and invasion of breast cancer cells by targeting tumor protein D52 (TPD52) Oncology Research. 2017;25:753–761. doi: 10.3727/096504016X14772342320617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [26].Catriona Y.L., Roel N.. The Wnt signaling pathway in development and disease. Annual Review of Cell Development Biology. 2014;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- [27].Komiya Y., Habas R.. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].He B., You L., Kazutsugu U., Xu Z.D., Amie Y.L., Maria M.. A monoclonal antibody against wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;1:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maciej W., Aleksandra P., Piotr G., Maria M., Andrzej K.B., Monika L.P.. Silencing of wnt-1 by siRNA induces apoptosis of MCF-7 human breast cancer cells. Cancer Biology and Therapy. 2008;2:268–274. doi: 10.4161/cbt.7.2.5300. [DOI] [PubMed] [Google Scholar]
- [30].Xue M.L, Ge Y.L., Zhang J.Y., Liu Y.C., Wang Q., Hou L.. Fucoidan inhibited 4T1 mouse breast cancer cell growth in vivo and vitro via downregulation of Wnt/β-catenin signaling. Nutrition and Cancer. 2013;3:460–468. doi: 10.1080/01635581.2013.757628. [DOI] [PubMed] [Google Scholar]
- [31].Jiang Q., He M., Guan S., Ma M.T., Wu H.Z., Yu Z.J.. microRNA-100 suppresses the migration and invasion of breast cancer cells by targeting FZD-8 and inhibiting Wnt/β-catenin signaling pathway. Tumor Biology. 2016;37:5001–5011. doi: 10.1007/s13277-015-4342-x. [DOI] [PubMed] [Google Scholar]
- [32].Xue M.L., Wang Q., Zhao J.L., Dong L.Y., Ge Y.L., Hou L.. Docosahexaenoic acid inhibited the Wnt/β-catenin pathway and suppressed breast cancer cells in vitro and in vivo. The Journal of Nutrition Biochemistry. 2014;25:104–110. doi: 10.1016/j.jnutbio.2013.09.008. [DOI] [PubMed] [Google Scholar]
- [33].Wang Z.Y., Li B., Zhou L., Yu S.B., Su Z.J., Song J.X.. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;113:13150. doi: 10.1073/pnas.1616336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yin X.D., Xiang T.X., Li L.L., Su X.W., Shu X.S., Luo X.R.. DACT1, an antagonist to Wnt/β-catenin signaling, suppresses tumor cell growth and is frequently silenced in breast cancer. Breast Cancer Research. 2013;15:R23. doi: 10.1186/bcr3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jiang G., Xiao X., Zeng Y., Nagabhushanam K., Majeed M., Xiao D.. Targeting beta-catenin signaling to induce apoptosis in human breast cancer cells by z-Guggulsterone and Gugulipid extract of Ayurvedic medicine plant Commiphora mukul. BMC Complementary and Alternative Medicine. 2013;13:1–12. doi: 10.1186/1472-6882-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arnold A., Papanikolaou A.. Cyclin D1 in breast cancer pathogenesis. Journal of Clinical Oncology. 2015;23:4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- [37].Velasco-Velazques M.A., Li Z., Casimiro M., Loro E., Homsi N., Pestell R.G.. Examining the role of cyclin D1 in breast cancer. Future Oncology. 2011;7:753–765. doi: 10.2217/fon.11.56. [DOI] [PubMed] [Google Scholar]
- [38].El-Hafez A.A., El A.S.A., Hasan B.. Cyclin D1 overexpression associates with favorable prognostic factors in invasive breast carcinoma. Cancer Biomarkers. 2012;12:149–154. doi: 10.3233/CBM-130303. [DOI] [PubMed] [Google Scholar]
- [39].Wang C.D., Yuan C.F., Bu Y.Q., Wu X.M., Wan J.Y., Zhang L.. Fangchinoline inhibits cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pacific Journal of Cancer Prevention. 2014;15:769–773. doi: 10.7314/apjcp.2014.15.2.769. [DOI] [PubMed] [Google Scholar]
- [40].Chandrashekhar D., Dattu P.Y., Reinhard W., Ramesh U.. Tumor protein D52 (isoform 3) contributes to prostate cancer cell growth via targeting nuclear factor-κB transactivation in LNCaP cells. Tumor Biology. 2017;39:1–16. doi: 10.1177/1010428317698382. [DOI] [PubMed] [Google Scholar]
- [41].Chandrashekhar D., Dattu P.Y., Reinhard W., Ramesh U.. Tumor protein D52 (isoform) contributes to prostate cancer cell growth via targeting nuclear factor-κB transactivation in LNCaP cells. Tumor Biology. 2017;39:101042831769838. doi: 10.1177/1010428317698382. [DOI] [PubMed] [Google Scholar]
- [42].Kotapalli S.S., Dasari C., Duscharla D., Karthik R., Kami R., Kasula M., Ummanni R.. All-trans-retinoic acid stimulates overexpression of tumor protein D52 (TPD52, isoform 3) and neuronal differentiation of IMR-32 cells. Journal of Cellular Biochemistry. 2017;118:4358–4369. doi: 10.1002/jcb.26090. [DOI] [PubMed] [Google Scholar]


