Fig. (1).
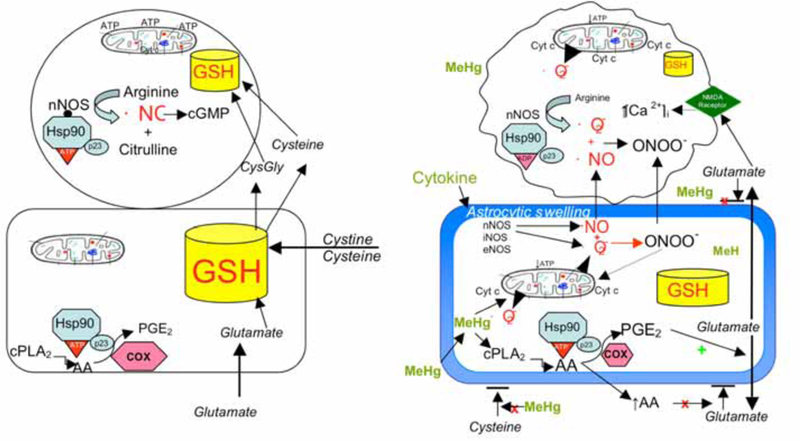
Panel A represents the normal interrelationship between astrocytes and neurons, in which astrocytes contain a larger pool of GSH (and other antioxidants) than is found in neurons. Astrocytes synthesize GSH from the uptake of cystine, cysteine and glutamate. Neuronal GSH concentrations are dependent largely on release of precursors from astrocytes. Healthy neurons express nNOS which, when bound to Hsp90 in the proper ATP conformation, produces ·NO which activates guanylate cyclase to produce cGMP for maintenance of important biological functions. Astrocytes do not express NOS but do produce PGE2, which is important for normal tissue homeostasis. Intact mitochondrial function and intracellular GSH levels in both cell types are important for normal ATP production and cell bioenergetics. Panel B depicts the alterations in astrocytic and neuronal cell signaling that occur with MeHg toxicity. MeHg induces astrocytic swelling triggering glutamate release, and inhibits uptake of cystine and cysteine, reducing astrocytic ability to synthesize GSH. MeHg also stimulates cPLA2 and AA release which stimulates increased production of PGE2 via PGES/p23 bound to Hsp90. PGE2 stimulates glutamate release and AA blocks reuptake, resulting in markedly increased levels of extracellular glutamate. Glutamate stimulates NMDA receptors on neurons increasing [Ca2+]I which causes activation of nNOS and mitochondrial dysfunction. MeHg (perhaps by induction of cytokines) induces astrocytes to express nNOS (and other NOS isoforms) which produce both ·NO and ·O2−. These ROS combine to form ONOO−, which together with NO diffuse to adjacent neurons increasing oxidant stress. This will markedly reduce intracellular GSH, especially in neurons. Increased ·NO, ONOO− and Ca2+ overload damages the mitochondrial ETC, resulting in reduced ATP formation, increased ·O2− formation, and cyt c release, all of which initiate the cascades leading to neuronal death.
