Figure 1. A model for B cell development, selection, and function after B cell depletion therapy.
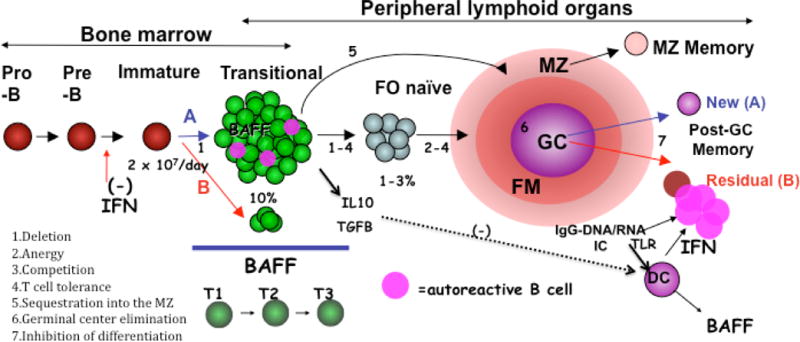
The outcome of BCD is going to depend on how well established autoimmunity is eradicated and how the immune system reconstitutes. B cells are continually generated in the bone marrow, and once rituximab is cleared can develop through well recognized stages (T=transitional, FO=follicular naïve, FM=follicular mantle, MZ=marginal zone, GC=germinal center) with defined tolerance check points as shown. Autoreactive B cells (depicted in pink) are deleted in the BM immature subsets, and cells in the transitional subsets undergo further selection, the stringency of which is determined in part by available BAFF (BAFF excess due to overproduction, peripheral lymphopenia, or reduced BM output reduces selective stringency). A favorable reconstitution profile (denoted A) will be characterized by an abundance of newly emerging transitional B cells in an environment that favors stringent negative selection of autoreactivity (high numbers of transitional B cells relative to BAFF). A non-favorable reconstitution profile (B in red) will be characterized by a higher fraction of residual memory B cells induced by an environment of TLR activation (via DNA and RNA containing immune complex activation of plasmacytoid dendritic cells), yielding large quantities of interferon and inhibition of new BM B cell lymphopoiesis. The outcome of BCD will also depend on the balance between protective (regulatory, anti-inflammatory) B cells and pathogenic (effector, pro-inflammatory) B cells and their corresponding cytokines. We postulate that physiologically, transitional cells predominantly produce IL10 (or TGFB) which in a normal environment exerts anti-inflammatory actions. This situation would be altered in autoimmune disease. Finally, BCDT may restore the physiological balance between protective and pathogenic B cell functions by creating an environment dominated by transitional cells with anti-inflammatory and tolerogenic functions and Treg inducing activity.
