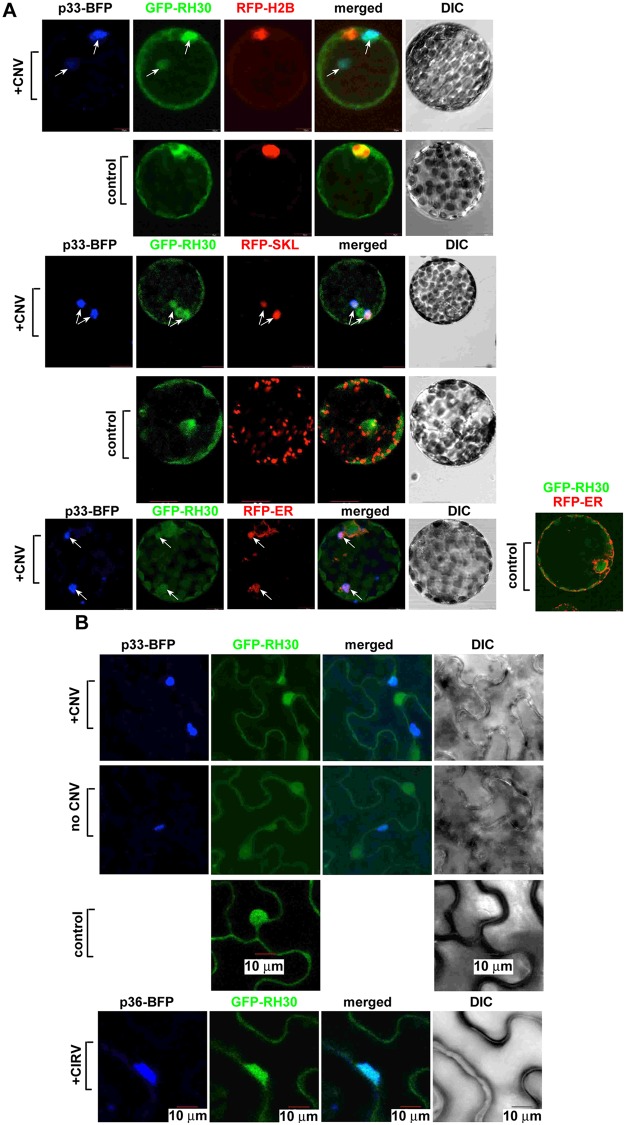
Fig 3. Confocal microscopy shows the retargeting of the mostly nuclear RH30 into the large replication compartment in plant protoplasts and whole plants infected with CNV.
(A) Most of RH30 is re-targeted into the replication compartment marked by the BFP-tagged p33 replication protein (pointed by arrows) in N. benthamiana protoplasts. Second panel: in the absence of viral components, GFP-tagged RH30 is mostly present in the nucleus, as marked by the histone protein (RFP-H2B). Third panel: The re-targeted GFP-RH30 is present in the viral replication compartment, marked by p33-BFP replication protein and RFP-SKL peroxisomal matrix marker. Arrows point at the viral replication compartment. Fourth panel: RH30 is not co-localized to the peroxisomes in the absence of tombusvirus replication. Fifth panel: The re-targeted GFP-RH30 is partially co-localized with the ER marker within the viral replication compartment, marked by p33-BFP replication protein. The leaves of N. benthamiana plants (transgenic plants expressing nucleus marker RFP-H2B or ER marker RFP-ER) were agro-infiltrated to express p33-BFP, GFP-RH30, and CNV20KSTOP gRNA. Leaves without the expression of p33-BFP and CNV20KSTOP gRNA were used as controls. The agro-infiltrated leaves were collected to isolate protoplasts for confocal imaging 2.5 days post agro-infiltration. Scale bars represent 10 μm. (B) Confocal microscopy images show co-localization of TBSV p33-BFP or CIRV p36-BFP replication proteins and the GFP-RH30 in planta. The large replication compartment was visualized via expression of TBSV p33-BFP or CIRV p36-BFP. Expression of the above proteins from the 35S promoter was done after co-agroinfiltration into N. benthamiana leaves. The leaves of N. benthamiana plants were agro-infiltrated to express TBSV p33-BFP or the CIRV p36-BFP, GFP-RH30, and CNV20KSTOP or CIRV gRNAs. Leaves without the expression of p33-BFP or p36-BFP and the viral RNAs were used as controls. The agro-infiltrated leaves were collected for confocal imaging 2.5 days post agro-infiltration. Scale bars represent 10 μm. Each experiment was repeated.