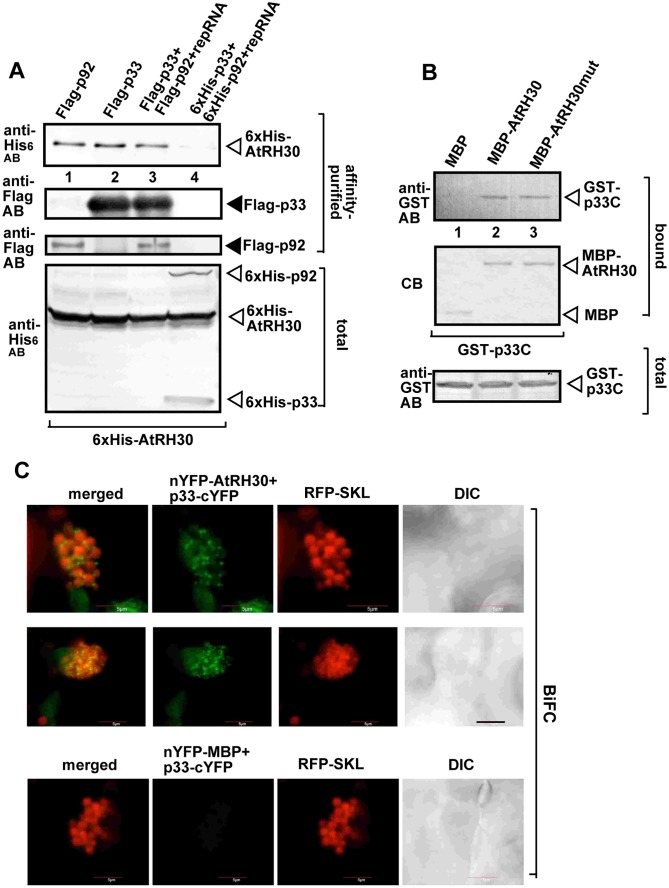
Fig 5. Co-purification of RH30 helicase with the viral replicase from membranous fraction of yeast.
(A) Co-purification of His6-tagged RH30 with Flag-p33 and Flag-p92pol replication proteins from subcellular membranes. Top panels: Western blot analysis of co-purified His6-RH30 (lanes 1, 2, and 3) with Flag-affinity purified replicase, Flag-p33 and Flag-p92pol replication proteins, respectively as shown. His6-p33, His6-p92pol and His6-RH30 were detected with anti-His antibody, while Flag-p33 and Flag-p92pol replication proteins were detected with anti-FLAG antibody. The negative control was from yeast expressing His6-RH30, His6-p33 and His6-p92pol purified in a FLAG-affinity column (lane 4). Bottom panel: blot of total His6-p33 and His6-p92pol and His6-RH30 in the total yeast extracts detected with anti-His antibody. (B) Pull-down assay including TBSV GST-p33 replication protein and the MBP-tagged RH30. Note that we used the soluble C-terminal region of TBSV p33 replication protein, which lacked the N-terminal sequence, including the trans-membrane TM domain. Top panel: Western blot analysis of the captured GST-p33C with the MBP-affinity purified MBP-RH30 or the helicase core mutant of RH30 (RH30mut, F416L) was performed with anti-His antibody. The negative control was MBP (lane 1). Middle panel: Coomassie-blue stained SDS-PAGE of the captured MBP-RH30 and MBP. Bottom panel: Western blot analysis of GST-p33C in total E. coli lysates. Each experiment was repeated three times. (C) Interactions between TBSV p33 replication protein and the RH30 helicase was detected by BiFC. The TBSV p33-cYFP replication protein and the nYFP-RH30 and the RFP-SKL peroxisomal marker protein were expressed via agro-infiltration. The merged image shows the efficient co-localization of the peroxisomal RFP-SKL with the BiFC signals, indicating that the interaction between the tombusvirus replication protein and the recruited RH30 helicase occurs in the large viral replication compartments, which consist of aggregated peroxisomes. Scale bars represent 5 μm.