Abstract
Background
The molecular classification of gastric cancer recognises two subtypes prone to immune checkpoint blockade: the microsatellite unstable and the Epstein-Barr virus (EBV)-related tumours. We aim to assess the concordance between immunohistochemistry and PCR for microsatellite status evaluation, and explore the value of microsatellite instability (MSI) and EBV as predictive survival factors.
Material and methods
We collected 246 consecutively diagnosed gastric cancer cases in all stages and evaluated the microsatellite status using immunohistochemistry for mismatched repair (MMR) proteins and PCR. EBV expression was studied through in situ hybridisation.
Results
Forty-five (18%) cases presented MSI and 13 (6%) were positive for EBV. MSI was associated with female sex, older age, distal location and distal non-diffuse type of the modified Lauren classification. EBV expression was most frequent in proximal location and proximal non-diffuse type. The sensitivity, specificity, positive predictive value and negative predictive value of immunohistochemistry for the microsatellite study were 91%, 98%, 91% and 98%, respectively. In the multivariate analysis, MSI was an independent predictor of favourable tumour-specific survival (TSS) in stages I–III (MSI: HR: 0.37, 95% CI 0.12 to 0.95, p=0.04).
Conclusions
The MSI status and the EBV expression should be incorporated in routine pathological report for two reasons. First, MSI defines a different pathological entity with a better outcome. Second, MSI and EBV may be useful biomarkers to identify patients who will respond to immune checkpoint blockade inhibitors. For this purpose, immunohistochemical study for MMR proteins and in situ hybridisation study for EBV evaluation are feasible and cost-effective methods.
Keywords: gastric cancer, epstein-barr virus, microsatellite instability
Key questions.
What is already known about this subject?
Recently, microsatellite instability (MSI) and Epstein-Barr virus (EBV) infection have proved to be useful biomarkers to identify patients with gastric cancer who will respond to immune checkpoint blockade inhibitors.
What does this study add?
Our study adds valuable information about MSI and EBV gastric cancer subtype characterisation and identification in daily practice.
According to our results, MSI in gastric cancer defines a different pathological entity with a better outcome (MSI: HR: 0.37, 95% CI 0.12 to 0.95, p=0.04).
For MSI evaluation in gastric cancer, immunohistochemical study for mismatched repair (MMR) proteins and PCR show an excellent concordance.
Immunohistochemical study for MMR proteins and in situ hybridisation (ISH) for EBV evaluation are feasible and cost-effective methods.
How might this impact on clinical practice?
There is an urgent need to improve risk stratification in patients with gastric cancer.
For this purpose, MSI and EBV are useful biomarkers and should be incorporated in routine pathological evaluation.
Our results proved IHC for MMR proteins and ISH study for EBV to be applicable in daily practice.
The results of both techniques significantly correlate with certain clinicopathological features and with the outcome.
Introduction
Gastric cancer (GC) was the global third leading cause of cancer mortality in 2015, responsible for 819 000 deaths.1 Significant advances in the understanding of the disease have been achieved as a result of comprehensive molecular and functional characterisations.2 3 The Cancer Genome Atlas (TCGA) classification established four main subgroups: tumours positive for Epstein-Barr virus (EBV-GC), microsatellite unstable tumours (MSI-GC), genomic stable tumours and tumours with chromosomal instability.2 Sporadic MSI-GC shows epigenetic silencing of MLH1 in the context of a CpG island methylator phenotype, causing a defective mismatched repair (MMR) mechanism, and the accumulation of insertion and deletion loops in gene coding regions responsible for important cell functions.4 5 The molecular classification proposed by Cristescu et al3 established four different subgroups regarding TCGA. However, both recognise MSI-GC as a special subgroup of gastric tumours. Although for the Cristescu et al classification EBV-GC was not a distinct entity, they noted the subgroup of TP53-activated tumours was enriched by EBV-positive cases.3 MSI-GC accounts for around 15%–30% of sporadic GCs, and EBV positivity has been reported in 10% of cases.4–6 The hypermutated nature of sporadic MSI-GC and the amplification of programmed death-ligand 1 (PD-L1) usual in EBV-GC make them liable for immune checkpoint blockade.7–9 MSI-GC is associated with a decreased response to fluorouracil-based chemotherapy, and most studies point microsatellite instability (MSI) as a predictor of a better cancer-specific survival.10–19 Therefore, the new American Joint Committee on Cancer (AJCC) staging manual (eighth edition) recommends MSI testing in both patients with GC and patients with colorectal cancer (CRC).20
Next-generation sequencing could be the most sensitive method for detecting MSI status. However, in the clinic, immunohistochemistry (IHC) expression of MMR proteins has gained acceptance as the most cost-effective method to detect MSI in CRC, showing an excellent concordance with the study through PCR. However, there are few studies exploring the concordance between immunohistochemistry and PCR for the MSI status in GC, and most differ widely on their design, number of nucleotides and proteins analysed.9 12 15–19 21–24 We built a series of 246 GCs to evaluate the MSI status using both immunohistochemistry for MMR proteins (MLH1, MSH2, MSH6 and PMS2) and PCR for five quasimonomorphic mononucleotide repeats (BAT-25, BAT-26, NR-21, NR-24 and NR-27) in all cases, to determine the most accurate procedure and the concordance between both methods. Finally, we study the Epstein-Barr virus-encoded small RNAs (EBER) expression through in situ hybridisation (ISH) to evaluate its relation with the MSI status and their clinical implications as predictive survival factors.
Materials and methods
Formalin-fixed, paraffin-embedded GC samples consecutively diagnosed as adenocarcinoma over a period of 14 years (2003–2017) were collected. Cases with depleted tissue were excluded. A total of 246 patients with GC were identified: 191 of them have undergone either total or partial gastrectomy, and in the remaining 55 endoscopic biopsies were the only available material. All the slides were reviewed by two pathologists (CM and SN). Each case was classified according to the Lauren classification for histological subtype, and the AJCC classification (eighth edition) for depth of invasion and involvement of regional lymph nodes.20 25 In order to avoid tumour heterogeneity bias, we selected the most representative paraffin block of each case and performed all the procedures (immunohistochemistry, ISH and DNA isolation) in the same material. Clinicopathological and follow-up information was collected from all of the participants.
Immunohistochemistry
Paraffin-embedded tumour sections of 3 μm in thickness were deparaffinised, and antigen retrieval was done with a pressure cooker in citrate buffer at high pH (Dako, Glostrup, Denmark). Immunohistochemical study was done using an automatised system Autostainer Link 48 (Dako) with monoclonal antibodies: MLH1 (clone IR079, dilution 1/100, Dako), MSH2 (clone IR085, dilution 1/100, Dako), MSH6 (clone IR086, dilution 1/100, Dako) and PMS2 (clone IR087, dilution 1/100, Dako). Only nuclear staining with or without cytoplasmic staining in tumour cells was considered positive. Peritumorous lymphocytes, stromal cells and non-neoplastic epithelial cells were used as internal control. Only the complete loss of nuclear staining with positive internal control was considered as loss of MMR protein expression. Normal expression was defined as the presence of nuclear staining in tumour cells, irrespective of the intensity.
ISH for EBER
ISH for EBV was done using the EBER-PNA EnVision flex probe (Dako). Paraffin-embedded tumour tissue sections of 3 μm in thickness were deparaffinised and treated with proteinase K for 10 min. Probes were applied followed by denaturation and hybridisation at 60°C (1 hour). The tissue sections were visualised using a rabbit antibody and revealed with HRP-FLEX Dako polymers. The intensity of staining (weak, moderate or intense) and the percentage of positive cells were recorded. Cases showing nuclear staining in at least 5% of tumour cells were considered positive for EBER.
Fluorescent multiplex PCR-based method for MSI status
Genomic DNA was isolated from three unstained 20 μm sections using a QIAamp DNA FFPE Tissue Kit (Qiagen) following the manufacturer’s instructions. The final DNA concentration was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, Delaware, USA) and subsequently stored at –20°C.
Five quasimonomorphic mononucleotide repeats—BAT-25, BAT-26, NR-21, NR-24 and NR-27—were coamplified in a single multiplex PCR (pentaplex) containing 100 ng sample DNA, 7.5 µL PCR master mix (2×) and 1.5 µL primer mix and 5 µL H20, following the manufacturer’s protocol (Multiplex PCR; Qiagen). The primers used were the following: BAT-25: 5 ‘TACCAGGTGGCAAAGGGCA3’- 5’ ‘TCTGCATTTTAACTATGG3’; BAT-26: 5 ‘CTGCGGTAATCAAGTTTTTAG3’- 5 ‘AACCATTCAACATTTTTAACCC3’; NR-21: 5 ‘GAGTCGCTGGCACAGTTCTA3’ −5 ‘CTGGTCACTCGCGTTTACAA3’; NR-24: 5 ‘GCTGAATTTTACCTCCTGAC3’−5 ‘ATTGTGCCATTGCATTCCAA3’ and NR-27: 5 ‘AACCATGCTTGCAAACCACT3’ −5 ‘CGATAATACTAGCAATGACC3’. The 5’antisense primer was labelled with a fluorescent dye using FAM for BAT- 26 and NR-21, NED for BAT-25 and NR-27, and VIC for NR-24 (ABI PRISM Primer Pairs, Applied Biosystems, Foster City, California). The allelic profiles were detected on an automated DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems) as we did previously.26 We classified a tumour as MSI when two or more markers showed instability.
Statistical analysis
Statistical analyses of data were carried out in the free software R environment, V.3.4.2 (Vienna, Austria; www.r-project.org). A p value of less than 0.05 was considered statistically significant. Comparison of clinical and pathological patients’ characteristics was done using the χ2 test, the Fisher’s exact test or the Wilcoxon test for qualitative and quantitative variables, respectively, prior to assessment of normality using the Shapiro-Wilk test. TSS was calculated from the diagnosis to the death of tumour-related causes or until the last known follow-up. Survival analyses were performed using Kaplan-Meier curves compared through log-rank test. Multivariate regression analysis was carried using Cox proportional hazards models with stepwise selection, including those variables significantly correlated with survival probability in the univariate analysis.
Results
Clinicopathological features
Features related to the MSI phenotype are summarised in table 1. In the molecular study, 45 out of 246 (18%) cases were MSI. MSI-GC was significantly associated with female sex, older age (mean 75 years), distal location (body and antrum) and distal non-diffuse modified Lauren classification (MLC). Histological subtype, pathological TNM (pTNM) classification, presence of lymph node metastasis and lymphovascular invasion showed no significant relation with MSI status.
Table 1.
Clinicopathological features related to MSI status
| MSS, n=201 (82%) | MSI, n=45 (18%) | P value | |
| Characteristics | |||
| Median age (range) | 68.1 (34–91) | 75.9 (52–95) | <0.001* |
| Gender, n (%) | |||
| Female | 57 (28.3) | 24 (53.3) | 0.001† |
| Male | 144 (71.6) | 21 (46.6) | |
| Location, n (%) | |||
| GEJ and cardia | 54 (26.8) | 5 (11.1) | 0.03† |
| Body and antrum | 141 (70.1) | 40 (88.8) | |
| Gastric pouch | 6 (2.9) | 0 | |
| Lauren histological classification, n (%) | |||
| Intestinal | 124 (61.6) | 30 (66.6) | 0.13† |
| Diffuse | 55 (27.3) | 7 (15.5) | |
| Mixed | 22 (10.9) | 8 (17.7) | |
| Tumour location-modified Lauren classification, n (%) | |||
| PND | 43 (21.3) | 4 (8.8) | 0.05† |
| D | 77 (38.3) | 15 (33.3) | |
| DND | 81 (40.3) | 26 (57.7) | |
| pTNM stage, n (%) | |||
| I | 35 (23) | 8 (20) | 0.40† |
| II | 44 (28.9) | 12 (30) | |
| III | 58 (38.1) | 19 (47.5) | |
| IV | 15 (9.8) | 1 (2.5) | |
| pN stage, n (%) | |||
| N0 | 62 (41.1) | 13 (33.3) | 0.06† |
| N1 | 23 (15.2) | 11 (28.2) | |
| N2 | 28 (18.5) | 9 (23.1) | |
| N3 | 38 (25.2) | 6 (15.4) | |
| Lymphovascular invasion, n (%) | |||
| Absent | 65 (43) | 18 (45) | 0.82† |
| Present | 86 (57) | 22 (55) | |
| EBER expression, n (%) | |||
| Positive | 10 (6) | 3 (7) | 0.74† |
| Negative | 158 (94) | 38 (93) | |
Significant p-values are shown in bold
*Wilcoxon test.
†2 test.
D, diffuse; DND, distal non-diffuse; EBER, Epstein-Barr virus-encoded small RNAs; GEJ, gastro-oesophageal junction; MSI, microsatellite instability; MSS, microsatellite stable; PND, proximal non-diffuse; pN, pathological N; pTNM, pathological TNM.
EBV expression was evaluable in 209 cases, and 13 of them (6%) were positive. Three (24%) cases showed expression in 5%–10% of tumour cells, 6 (46%) in more than 80%, and in the remaining 4 (30%) the positivity ranged from 10% to 80% of cells (online supplementary figure S1).
esmoopen-2018-000470supp001.jpg (5.3MB, jpg)
The intensity of staining was low in 4 (31%) cases, moderate in 2 (15%) and strong in 7 (54%). EBV expression was most frequent in proximal location and proximal non-diffuse type of the MLC (p=0.004 in both cases). Only 3 out of 13 (23%) EBV-positive cases were also MSI. Age, sex, MSI status, pTNM classification, histological grade, histological subtype, presence of lymph node metastasis and lymphovascular invasion showed no significant relation with EBER expression (table 2).
Table 2.
Clinicopathological features related to EBV expression
| EBV-positive, n=13 (6%) | EBV-negative, n=196 (94%) | P value | |
| Characteristics | |||
| Median age (range) | 66.7 (38–87) | 69.9 (64–95) | 0.47* |
| Gender, n (%) | |||
| Female | 3 (23) | 69 (35) | 0.37† |
| Male | 10 (77) | 127 (65) | |
| Location, n (%) | |||
| GEJ and cardia | 8 (62) | 43 (22) | 0.005† |
| Body and antrum | 5 (38) | 147 (75) | |
| Gastric pouch | 0 | 6 (3) | |
| Lauren histological classification, n (%) | |||
| Intestinal | 10 (77) | 120 (61.2) | 0.52† |
| Diffuse | 2 (15.3) | 50 (25.51) | |
| Mixed | 1 (7.7) | 26 (13.27) | |
| Tumour location-modified Lauren classification, n (%) | |||
| PND | 7 (54) | 33 (16.8) | 0.01† |
| D | 3 (23) | 76 (38.8) | |
| DND | 3 (23) | 87 (44.4) | |
| pTNM stage, n (%) | |||
| I | 1 (8.3) | 37 (22.4) | 0.66† |
| II | 5 (41.6) | 49 (29.7) | |
| III | 5 (41.6) | 65 (39.4) | |
| IV | 1 (8.3) | 14 (8.5) | |
| pN stage, n (%) | |||
| N0 | 4 (33.3) | 66 (40) | 0.73† |
| N1 | 3 (25) | 30 (18.2) | |
| N2 | 2 (16.7) | 30 (18.2) | |
| N3 | 3 (25) | 39 (23.6) | |
| Lymphovascular invasion, n (%) | |||
| Absent | 8 (62) | 71 (36.4) | 0.11† |
| Present | 4 (38) | 94 (35.4) | |
Significant p-values are shown in bold
*Wilcoxon test.
†Fisher’s exact test.
D, diffuse; DND, distal non-diffuse; EBV, Epstein-Barr virus; GEJ, gastro-oesophageal junction; PND, proximal non-diffuse; pN, pathological N; pTNM, pathological TNM.
Clinical information regarding therapeutic strategy in patients with localised disease was available in 146 patients. Ninety (62%) of them underwent surgery alone and 56 (38%) received perioperative platinum-based chemotherapy. Thirty-five (63%) patients completed all the preoperative treatments. However, only 12.6% of the 56 patients who received perioperatory treatment were able to complete the whole preoperative and postoperative treatments. Fifty (89%) patients received a combination of 5-fluorouracil/capecitabine with oxaliplatin.
Immunohistochemistry and PCR results
Forty-five cases (18%) showed loss of MMR protein expression: 91% (n=41/45) lost MLH1 and PMS2, and 9% (n=5/45) MSH2 and MSH6. In the PCR study, 40 (89%) cases were unstable for the five nucleotides analysed, and the remaining 5 cases were unstable for at least three markers. NR-21 was constantly unstable in all MSI tumours (100%). BAT-26, NR-27, NR-24 and BAT-25 were unstable in 98% (n=44), 98% (n=44), 95% (n=43) and 91% (n=41) of cases, respectively. Four MMR-negative cases (three of them negative for MLH1/PMS2 and one negative for MSH6/MSH2) resulted microsatellite stable in the molecular evaluation. Conversely, four MSI cases (three unstable for the five nucleotides, and one unstable for NR-21, NR-27 and BAT-26) retained the immunohistochemical expression of MMR proteins (online supplementary table S1 and figure S2). The sensitivity, specificity, positive predictive value and negative predictive value of the MMR immunostaining for the MSI status were 91%, 98%, 91% and 98%, respectively.
esmoopen-2018-000470supp005.pdf (11.5KB, pdf)
esmoopen-2018-000470supp002.png (49.9KB, png)
Survival analysis
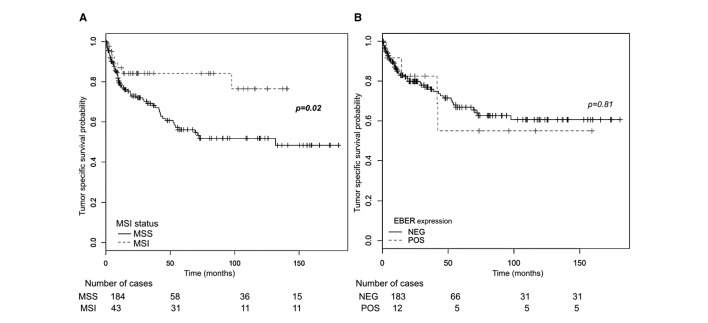
The median follow-up period was 38.5 months (range: 3–180 months). In the survival analysis of patients in stages I–III, patients with MSI-GC showed a significantly lower risk of cancer-related death (HR: 0.43, 95% CI 0.05 to 0.10, p=0.03) (table 3). MSI status was also analysed by stage (online supplementary figure S3). Patients with MSI who did not receive perioperative treatment did better than those who underwent perioperative chemotherapy, particularly in stage II, although this difference did not reach statistical significance, probably due to the retrospective nature of this analysis (online supplementary figure S4). The presence of nodal metastasis, distal location and the distal non-diffuse subtype of the MLC also influenced TSS probability (figure 1). EBER expression did not reach a significant relation with survival probability.
Table 3.
Univariate analysis for tumour-specific survival in stages I–III
| HR | 95% CI | P value | |
| Gender (male) | 0.91 | 0.55 to 1.50 | 0.71 |
| MSI | 0.43 | 0.05 to 0.10 | 0.03 |
| EBER-positive | 1.13 | 0.40 to 3.14 | 0.81 |
| pT3–4 vs 1–2 | 1.67 | 0.78 to 3.57 | 0.18 |
| Nodal metastasis | 2.56 | 1.17 to 5.59 | 0.01 |
| Distal location | 0.53 | 0.32 to 0.86 | 0.01 |
| LV invasion | 1.41 | 0.70 to 2.86 | 0.32 |
| Histological subtype | |||
| Diffuse (vs intestinal) | 1.00 | 0.57 to 1.75 | 0.98 |
| Mixed (vs intestinal) | 0.92 | 0.39 to 2.15 | 0.84 |
| Tumour location-modified Lauren classification | |||
| D (vs PND) | 0.58 | 0.32 to 1.03 | 0.06 |
| DND (vs PND) | 0.42 | 0.23 to 0.75 | 0.003 |
Significant p-values are shown in bold
D, diffuse; DND, distal non-diffuse; EBER, Epstein-Barr virus-encoded small RNAs; LV, lymphovascular; MSI, microsatellite instability; PND, proximal non-diffuse.
Figure 1.
Kaplan-Meier survival curves for tumour-specific survival (TSS). (A) In stages I–III, TSS probability was significantly higher than in microsatellite stable cases. (B) No significant differences in TSS probability were observed between EBER-positive and EBER-negative cases. EBER, Epstein-Barr virus-encoded small RNAs; MSI, microsatellite instability; MSS, microsatellite stable; NEG, negative; POS, positive.
esmoopen-2018-000470supp003.png (181.4KB, png)
esmoopen-2018-000470supp004.png (134.7KB, png)
In the Cox multivariate analysis of patients in stages I–III, the most important factor for TSS was the presence of nodal metastasis (HR: 3.33, 95% CI 1.49 to 7.43, p=0.003). The MSI status and the presence of nodal metastases were the only independent factors influencing TSS. Patients with MSI-GC showed a significantly lower risk for tumour mortality (MSI: HR: 0.37, 95% CI 0.12 to 0.95, p=0.04) (table 4).
Table 4.
Multivariate analysis for tumour-specific survival in stages I–III
| HR | 95% CI | P value | |
| MSI | 0.37 | 0.12 to 0.95 | 0.04 |
| Nodal metastasis | 3.33 | 1.49 to 7.43 | 0.003 |
| Distal location | 1.55 | 0.18 to 12.72 | 0.68 |
| Tumour location-modified Lauren classification | |||
| D (vs PND) | 0.17 | 0.02 to 1.38 | 0.09 |
| DND (vs PND) | 0.71 | 0.02 to 2.57 | 0.25 |
Significant p-values are shown in bold
D, diffuse; DND, distal non-diffuse; MSI, microsatellite instability; PND, proximal non-diffuse.
Discussion
GC is a heterogeneous disease and the TCGA classification has improved our understanding of the pathogenesis and its most important molecular drivers. However, the lack of strong evidence for the clinical implications of each subtype, mostly because of the insufficient follow-up period, has limited the translation of the results to clinical practice.2 Although the MSI and the EBV subtypes of GC in the TCGA classification are defined by comprehensive multiomics analysis, most studies consider the immunohistochemical evaluation of the MMR proteins and the hybridisation study for EBER as surrogate markers to identify each subtype.8–10 24 27 28 However, there are few studies in the literature analysing GC for the correlation between the immunohistochemical expression of MMR proteins and the fluorescent multiplex markers by PCR.12 15 17 21 22 27 28 Most of the published studies follow the international consensus meeting proposed in 1997, the ‘Bethesda panel’, which recommends the use of five markers for the uniform analysis of MSI in CRC.29 These included two mononucleotide repeats (BAT-25 and BAT-26) and three dinucleotides (D5S346, D2S123 and D17S250) repeats. However, the sensitivity and specificity of this panel were questioned in the revised Bethesda guidelines for Lynch syndrome.30 Dinucleotide repeats are also highly polymorphic, and their use in MSI screening requires the analysis of the corresponding normal tissue. For all these reasons, the revised Bethesda guidelines recommend the addition of more mononucleotide markers, and highlight the need for further validation of MSI testing in tumour types other than colorectal and endometrial carcinoma.30 The description of quasimonomorphic mononucleotides, showing homozygous or quasi-homozygous state, with only minor size variations, allowed the MSI study in the absence of matching normal DNA. In our series, NR-21 was the most efficient of these markers, constantly unstable in all MSI tumours by PCR. According to our results, the agreement between the immunohistochemical study and the PCR for the MSI status in GC is excellent, and as in CRC the PCR may be used only when the immunohistochemistry is not conclusive.
Eighteen per cent of our series showed MSI, a frequency included within the range published in the literature.3 4 10–13 We found MSI-GC to be more frequent in women and older patients in concordance with the results of the TCGA classification. Unlike the TCGA work, in which MSI was not associated to a special tumour location, we found MSI-GC to be more frequent in distal locations and in the distal non-diffuse subtypes of the MLC. This relation between MSI and distal location has been described before in the molecular classification proposed by Cristescu et al and has been reproduced in other series.3 11 12 17 22 24 31 The relation between this feature and Helicobacter pylori infection, which is the most important driving force in the development of the distal non-diffuse GC, requires further research.11 12 17 22 23 In our study, MSI was significantly associated with a reduced risk of mortality in multivariate analysis, independently of other factors such as clinical stage or nodal metastasis. For these reasons, we consider that MSI testing should be included in clinical practice due to its prognosis implications in patients with GC. Moreover, MSI may aid valuable information to avoid neoadjuvant chemotherapy in patients with limited locoregional disease or to consider immunotherapy when relapsing or presenting with advanced stages.32–36
Six per cent of our cases were positive to EBV. This proportion is within the lower limit of the reported prevalence of this subtype. Most of our EBV-positive cases were localised in the gastro-oesophageal junction and cardias. However, other authors found EBV-GC to be associated with distal location.24 Contrary to the TCGA classification, EBV expression showed no gender preference in our series. Larger series are needed to better characterise the demographic features of this group of tumours. We have identified three EBV-positive cases with defective MMR mechanism confirmed by both immunohistochemistry and PCR. It has been described that EBV-positive GC and MSI-GC are mutually exclusive when analysed by comprehensive genomic analysis. However, in our series, we used PCR and IHC for determining MSI status and ISH to find expression of EBV. These methods could be certainly less sensitive, and therefore more limited to properly define the true molecular status. In these three mentioned cases, EBER was expressed in 10%, 20% and 100% of tumour cells, respectively. Probably, the lack of a diffuse staining for EBER in all tumour cells in most MSI cases may lead to consider them as negative for EBV. However, there is no a clear cut-off for defining positivity in the ISH study for EBER, and most published studies consider the nuclear staining in tumour cells as ‘positive’ without any reference to the intensity or the percentage of cells.19 23 27
Although there is no relation with different outcomes for EBV-related tumours, it presents very specific molecular features. The main reported molecular alterations in EBV-GC include hypermethylation of the promoter region of the gene CDKN2A, as well as several mutations in genes such as phosphatidylinositol-3-kinase catalytic subunit alpha (PIK3CA) observed in about 5%–10%. Furthermore, Janus kinase 2, PD-L1/L2 and their receptors (PD-1/2) are frequently overexpressed in EBV-GC.2 37 These alterations have important therapeutic implications. Indeed, PIK3CA mutations lead to constitutive activation of its pathway even in the absence of growth factors,38 causing acquired resistance to inhibitors such as anti-p110 and anti-protein kinase B (PKB or AKTs).39
PD-1/PD-L1 have been reported to be significantly overexpressed in MSI and EBV-GC subtypes, and recently a high sensitivity to pembrolizumab, a checkpoint inhibitor, has been observed in these subtypes in a prospective phase II clinical trial.40–44
The main limitations of our study are its retrospective and exploratory design, and the relatively small proportion of MSI and EVB-positive cases, which make difficult the stratification analysis of survival by clinical stages.
In conclusion, MSI status and EBV expression should be incorporated in routine pathological report for two reasons. First, MSI defines a different pathological entity with a better outcome. Second, MSI and EBV may be useful predictive biomarkers to identify patients responding to immune checkpoint inhibitors. For this purpose, immunohistochemical study for MMR proteins and ISH study for EBV evaluation are feasible and cost-effective methods.
Footnotes
CM-C and TF-K contributed equally.
Funding: This study was funded by Instituto de Salud Carlos III (Grant no: PI15/02180, PI16/00395).
Competing interests: None declared.
Patient consent for publication: All study subjects gave their written informed consent.
Ethics approval: The study protocol was approved by the Ethics Board at the INCLIVA Biomedical Research Institute.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. . Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and Disability-Adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol 2017;3:524–48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristescu R, Lee J, Nebozhyn M, et al. . Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. 10.1038/nm.3850 [DOI] [PubMed] [Google Scholar]
- 4.Lee HS, Kim WH, Kwak Y, et al. . Molecular testing for gastrointestinal cancer. J Pathol Transl Med 2017;51:103–21. 10.4132/jptm.2017.01.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balmaña J, Balaguer F, Cervantes A, et al. . Familial risk-colorectal cancer: ESMO clinical practice guidelines. Annals of Oncology 2013;24(suppl 6):vi73–80. 10.1093/annonc/mdt209 [DOI] [PubMed] [Google Scholar]
- 6.SHINOZAKI-USHIKU AYA, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol 2015;46:1421–34. 10.3892/ijo.2015.2856 [DOI] [PubMed] [Google Scholar]
- 7.Kim BJ, Jang HJ, Kim HS, et al. . Current status of immune checkpoint inhibitors in gastrointestinal cancers. J. Cancer 2017;8:1460–5. 10.7150/jca.18470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oki E, Kakeji Y, Zhao Y, et al. . Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol 2009;16:2510–5. 10.1245/s10434-009-0580-8 [DOI] [PubMed] [Google Scholar]
- 9.An JY, Kim H, Cheong J-H, et al. . Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer 2012;131:505–11. 10.1002/ijc.26399 [DOI] [PubMed] [Google Scholar]
- 10.Marrelli D, Polom K, Pascale V, et al. . Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric cancer. Ann Surg Oncol 2016;23:943–50. 10.1245/s10434-015-4931-3 [DOI] [PubMed] [Google Scholar]
- 11.Kim J-Y, Shin NR, Kim A, et al. . Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol 2013;47:28–35. 10.4132/KoreanJPathol.2013.47.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HS, Choi SI, Lee HK, et al. . Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol 2002;15:632–40. 10.1038/modpathol.3880578 [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Li Z, Wang Y, et al. . Microsatellite instability and survival in gastric cancer: a systematic review and meta-analysis. Mol Clin Oncol 2015;3:699–705. 10.3892/mco.2015.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polom K, Marrelli D, Roviello G, et al. . Molecular key to understand the gastric cancer biology in elderly patients-The role of microsatellite instability. J Surg Oncol 2017;115:344–50. 10.1002/jso.24513 [DOI] [PubMed] [Google Scholar]
- 15.Beghelli S, de Manzoni G, Barbi S, et al. . Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 2006;139:347–56. 10.1016/j.surg.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 16.Chiaravalli AM, et al. Histotype-based prognostic classification of gastric cancer. WJG 2012;18:896–904. 10.3748/wjg.v18.i9.896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falchetti M, Saieva C, Lupi R, et al. . Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Human Pathology 2008;39:925–32. 10.1016/j.humpath.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 18.Fang W, Chang S, Lan Y, et al. . Molecular and survival differences between familial and sporadic gastric cancers. Biomed Res Int 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grogg KL, Lohse CM, Pankratz VS, et al. . Lymphocyte-Rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol 2003;16:641–51. 10.1097/01.MP.0000076980.73826.C0 [DOI] [PubMed] [Google Scholar]
- 20.Amin M, Edge S, Greene F, et al. . AJCC cancer staging manual. 8th edn New York: Springer, 2017: 203–74. [Google Scholar]
- 21.Bacani J, Zwingerman R, Di Nicola N, et al. . Tumor microsatellite instability in early onset gastric cancer. The Journal of Molecular Diagnostics 2005;7:465–77. 10.1016/S1525-1578(10)60577-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo HM, Chang YS, Joo SH, et al. . Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J. Surg. Oncol. 2009;99:143–7. 10.1002/jso.21220 [DOI] [PubMed] [Google Scholar]
- 23.Solcia E, Klersy C, Mastracci L, et al. . A combined histologic and molecular approach identifies three groups of gastric cancer with different prognosis. Virchows Arch 2009;455:197–211. 10.1007/s00428-009-0813-z [DOI] [PubMed] [Google Scholar]
- 24.Mathiak M, Warneke V, Behrens H, et al. . Clinicopathologic characteristics of microsatellite instable gastric carcinomas revisited : urgent need for standardization. Appl Immunohistochem Mol Morphol 2017;1:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneiro F. Classification of gastric carcinomas. Current Diagnostic Pathology 1997;4:51–9. 10.1016/S0968-6053(97)80008-7 [DOI] [Google Scholar]
- 26.Llorca-Cardeñosa MJ, Fleitas T, Ibarrola-Villava M, et al. . Epigenetic changes in localized gastric cancer: the role of RUNX3 in tumor progression and the immune microenvironment. Oncotarget 2016;7:63424–36. 10.18632/oncotarget.11520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Kim EK, Kim YH, et al. . Epstein–Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer 2016;19:1041–51. 10.1007/s10120-015-0565-1 [DOI] [PubMed] [Google Scholar]
- 28.Bae YS, Kim H, Noh SH, et al. . Usefulness of immunohistochemistry for microsatellite instability screening in gastric cancer. Gut Liver 2015;9:629–35. 10.5009/gnl15133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boland C, Thibodeau S, Hamilton S, et al. . A national Cancer Institute workshop on microsatellite instability for cancer detection and famitial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 30.Umar A, Boland CR, Terdiman JP, et al. . Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. JNCI Journal of the National Cancer Institute 2004;96:261–8. 10.1093/jnci/djh034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Chung W, Lee K, et al. . Association between genetic instability and Helicobacter pylori infection in gastric epithelial dysplasia. Gastroenterol Res Pract 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janjigian YY, Sanchez-Vega F, Jonsson P, et al. . Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018;8:49–58. 10.1158/2159-8290.CD-17-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polom K, Marano L, Marrelli D, et al. . Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br J Surg 2018;105:159–67. 10.1002/bjs.10663 [DOI] [PubMed] [Google Scholar]
- 34.Polom K, Marrelli D, Smyth EC, et al. . The role of microsatellite instability in positive margin gastric cancer patients. Surg Innov 2018;25:99–104. 10.1177/1553350617751461 [DOI] [PubMed] [Google Scholar]
- 35.Pereira M, Ramos M, Faraj S, et al. . Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. J Surg Oncol 2018;117:829–39. 10.1002/jso.25022 [DOI] [PubMed] [Google Scholar]
- 36.Smyth E, Wotherspoon A, Peckitt C, et al. . Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical Research Council adjuvant gastric infusional chemotherapy (magic) trial. JAMA Oncol 2017;3:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derks S, Liao X, Chiaravalli AM, et al. . Abundant PD-L1 expression in Epstein-Barr virus-infected gastric cancers. Oncotarget 2016;7:32925–32. 10.18632/oncotarget.9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J-F, et al. Up-regulation of PIK3CA promotes metastasis in gastric carcinoma. World J Gastroenterol 2010;16:4986–91. 10.3748/wjg.v16.i39.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klempner SJ, Myers AP, Cantley LC. What a Tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discovery 2013;3:1345–54. 10.1158/2159-8290.CD-13-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hewitt LC, Inam IZ, Saito Y, et al. . Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-centre study. European Journal of Cancer 2018;94:104–14. 10.1016/j.ejca.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naseem M, Barzi A, Brezden-Masley C, et al. . Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treatment Reviews 2018;66:15–22. 10.1016/j.ctrv.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang Y-K, Boku N, Satoh T, et al. . Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 2017;390:2461–71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 43.Bang Y-J, Muro K, Fuchs CS, et al. . KEYNOTE-059 cohort 2: safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. JCO 2017;35(15_suppl):4012 10.1200/JCO.2017.35.15_suppl.4012 [DOI] [Google Scholar]
- 44.Kim ST, Cristescu R, Bass AJ, et al. . Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–58. 10.1038/s41591-018-0101-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000470supp001.jpg (5.3MB, jpg)
esmoopen-2018-000470supp005.pdf (11.5KB, pdf)
esmoopen-2018-000470supp002.png (49.9KB, png)
esmoopen-2018-000470supp003.png (181.4KB, png)
esmoopen-2018-000470supp004.png (134.7KB, png)