Figure 4. Renal-clearable AuNPs enhanced body clearance and minimized toxicity of the “off-target” DOX. a-d),
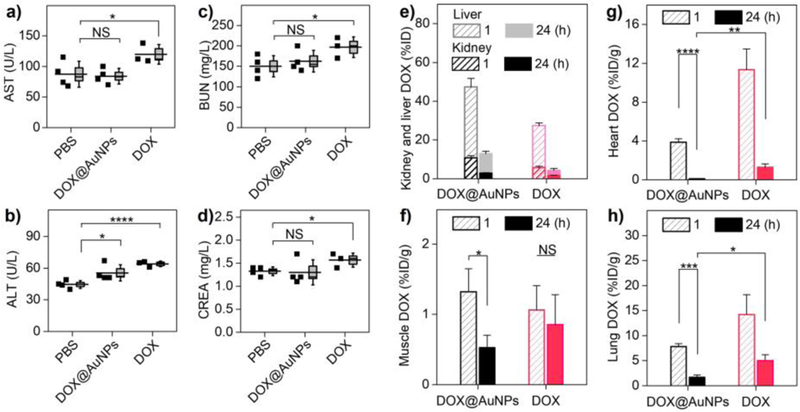
Blood chemistry analysis after successive treatments, indicating the acute toxicity of drug to liver function (aspartate transaminase, AST (a), alanine aminotransferase, ALT (b)) and kidney function (blood urea nitrogen, BUN (c), creatinine, CREA (d)). *P<0.05, ****P<0.0001, NS, not significant (n = 4 for DOX@AuNPs and PBS; n = 3 for free DOX, where one animal died prior to the end of the study). e) DOX distribution in liver and kidney for the injected DOX@AuNPs and free DOX (n = 3). f) Muscle DOX distribution at 1 and 24 h post-treatment (n = 3). *P<0.05. g-h) DOX distribution in vital organs as heart (g) and lungs (h) (n = 3). *P<0.05, **P<0.01, ***P<0.005, ****P<0.0001 (Student’s t-test).
