Figure 3. Chemical structures of current hydrophobic and water-soluble D-A-D fluorophores.
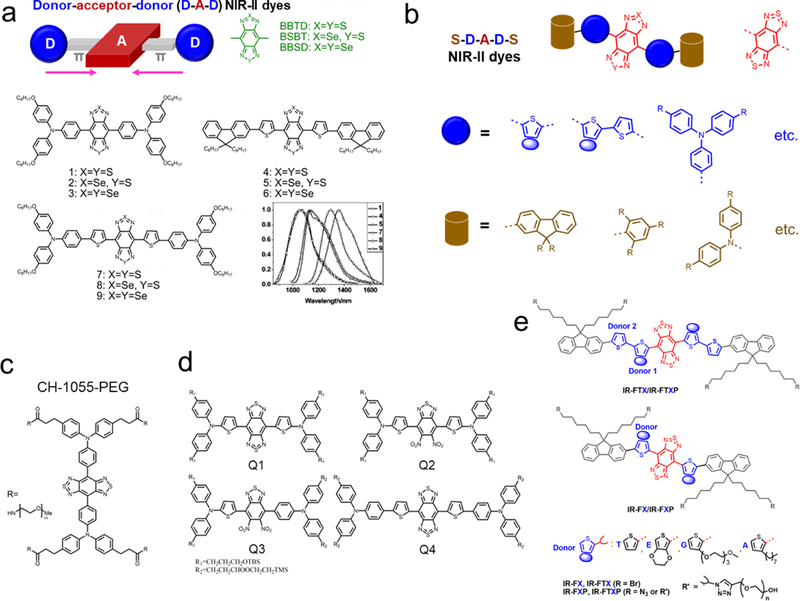
a) Peak regulation (from 1000 to 1400 nm) of hydrophobic D-A-D dyes with tuning both acceptors and donors. A typical guidance of chemical structure design is reported for D-A-D fluorophores with three acceptors, and the selective combination produced fluorophores 1–9 with tunable emission spectra in toluene. Reprinted with permission from Ref. [50][51]. b) The general structure of recently reported D-A-D/S-D-A-D-S dyes for NIR-II bioimaging. c) The first water-soluble NIR-II dye is CH1055-PEG. Reprinted with permission from Ref. [88]. d) Design of NIR-II dyes based on CH1055 scaffold and the chemical structures of Q1-Q4. Reprinted with permission from Ref. [92]. e) Schematic illustration of the design of bright molecular fluorophores with either single or double-donor optimization produced several key fluorophores.[18, 89, 93, 94] Reprinted with permission from Ref. [95]. To avoid repetition, please also check other structures in Figure 6,[72] 8[98, 101, 102], 10.[91] and Table 1.
