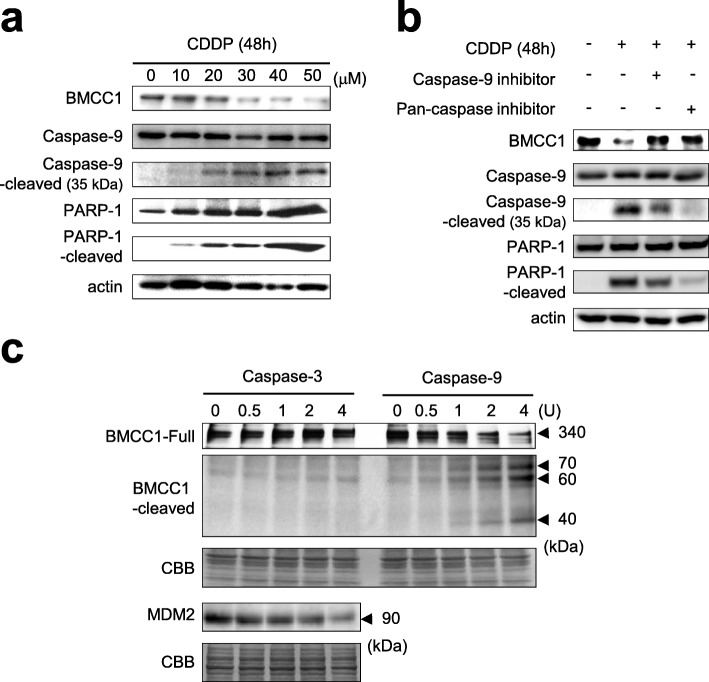
Fig. 5.
Cleavage of full-length BMCC1 during apoptosis was linked with activation of caspase-9. a Reduction of full-length BMCC1 was detected in apoptotic cells triggered by CDDP. SK-N-AS cells were treated with CDDP of various concentrations (0 to 50 μM). At 48 h after treatment, the harvested cells were immunoblotted. b Reduced amount of full-length BMCC1 by CDDP treatment was restored by inhibition of caspase-9. SK-N-AS cells were treated with 40 μM of CDDP for 24 h and cultured further in the presence or absence of 50 μM caspase inhibitors for 24 h. Harvested cells were analyzed by immunoblotting. c Cleavage of full-length BMCC1 by caspase-9 and not caspase-3 in vitro. Biotin-labeled full-length BMCC1 was synthesized using the TNT system and incubated with an active form of recombinant caspase-3 or caspase-9 at 37 °C for one hour. Protein degradation was detected using Streptavidin-HRP. Arrows indicate the full-length BMCC1 and three caspase-9-cleaved small bands. CBB staining of the gel was used as a loading control. MDM2 was cleaved by caspase-3 and used as a positive control of caspase-3 treatment