Abstract
Objective
To test for interactions between APOE ε4 genotype and lifestyle factors on worse cognitive abilities in UK Biobank.
Methods
Using UK Biobank cohort data, we tested for interactions between APOE ε4 allele presence, lifestyle factors of alcohol intake, smoking, total physical activity and obesity, and sex, on cognitive tests of reasoning, information processing speed, and executive function (n range = 70,988–324,725 depending on the test). We statistically adjusted for potential confounders of age, sex, deprivation, cardiometabolic conditions, and educational attainment.
Results
There were significant associations between APOE ε4 and worse cognitive abilities, independent of potential confounders, and between lifestyle risk factors and worse cognitive abilities; however, there were no interactions at multiple correction-adjusted p < 0.05, against our hypotheses.
Conclusions
Our results do not provide support for the idea that ε4 genotype increases vulnerability to the negative effects of lifestyle risk factors on cognitive ability, but rather support a primarily outright association between APOE ε4 genotype and worse cognitive ability.
There is some evidence that associations between known lifestyle-based risk factors for worse cognitive abilities—for example, diabetes,1 stress,2 traumatic brain injury,3 lower exercise,4 air pollution,5 and in some conditions female sex6,7—are larger in terms of effect size in people who possess an APOE ε4 allele (vs possessing nonrisk ε2 or ε3 alleles). With regards dementia as an outcome, there are similar findings for physical activity, dietary fat, alcohol intake, and smoking.8 Essentially, people with the ε4 allele may be more vulnerable to the effects of lifestyle risk factors on cognitive faculties. The potential biological rationale for this is that the APOE locus moderates lipid metabolism, which influences brain-relevant factors like white matter myelination and neuronal repair, meaning ε4 carriers may be more frail and vulnerable to the negative effects of suboptimal lifestyle risk factors.9,10 There have been instances of null results, however.11 It is also possible that there is a degree of file-drawer where null results are less likely to be published.12 There have been few large-scale systematic investigations into whether APOE ε4 interacts with lifestyle risk factors associated with worse cognitive abilities in a single cohort with a standard methodologic procedure.
UK Biobank is a large general population cohort with approximately 502,000 participants.13 All participants have baseline medical, cognitive, and sociodemographic data, and genetic data. We hypothesized that there would be a significant statistical interaction where known lifestyle factors would have larger associations with cognitive abilities in people who possessed APOE ε4 genotype (vs non-ε4).
Methodology
Study design and participants
The UK Biobank cohort is a large prospective general population cohort where baseline assessment took place between 2006 and 2010 in 22 assessment centres.13 In total, 502,628 participants aged 40–70 years were recruited from the general population. Invitation letters were sent to eligible adults registered with the NHS and living within 25 miles of a study assessment center. Participants completed a comprehensive touchscreen questionnaire including sociodemographic characteristics, physical and mental health, and a brief battery of cognitive tests. Across 2014–2015, participants who had provided an email address were invited to complete a remote, web-based questionnaire including cognitive tests. The project was completed using application number 17689 (PI: Dr. Lyall).
Cognitive assessment
At baseline assessment, participants completed 5 tests of cognitive ability, which were novel and computerized. We have described these in detail in an open-access report.14 For the current study, we focused on the 2 tests that showed acceptable intraparticipant stability across on average 4 years (intraclass r range = 0.54–0.65). In the first test, most participants completed a timed test of symbol matching, like the common card game Snap, hereafter referred to as reaction time (RT). The second test was a task with 13 logic/reasoning-type questions and a 2-minute time limit, labeled as fluid intelligence and referred to here simply as reasoning.15 The maximum score is 13. The reasoning task was only added to the battery partway through the baseline assessment phase and so around ∼150,000 participants completed it.
We did not examine the baseline tests of pairs matching, prospective memory, or numeric memory. The pairs-matching task was markedly zero-inflated (indicating floor effect) and did not show good longitudinal stability in ∼20,000 with repeat data (r < 0.2 across 4 years on average); prospective memory had around 94% overall success rate and thus had a degree of ceiling effect, and numeric memory was only completed by around 48,000 overall and did not have longitudinal data to suggest good reliability. These considerations have been described previously.14
After baseline assessment (2006–2010), between 2014 and 2015 participants were invited to complete a web-based questionnaire, where responders completed, among other things, web-based versions of 2 well-known cognitive tasks: Trail-Making Test A/B (TMT-A and TMT-B; processing speed and speed/executive function, respectively) and Digit Symbol Substitution Test (DSST) (executive function), each sensitive to the effects of cognitive ageing.16,17 Independent studies have shown good correlation between computerized vs paper-and-pen versions of the tests.18,19
Sociodemographic and medical data
Participants were asked during the baseline assessment about any previous or current cardiometabolic conditions that had been diagnosed by their doctor. Specifically, participants were asked whether their doctor had diagnosed myocardial infarction, angina, stroke, hypertension, or diabetes. We defined coronary heart disease (CHD) as either myocardial infarction or angina. We excluded participants who stated only “prefer not to answer.”
Participants reported their highest educational attainment and this was recoded into a simpler college/university degree vs no degree variable. Townsend20 deprivation indices were derived from postcode of residence. This provides an area-based measure of socioeconomic deprivation derived from aggregated data on car ownership, household overcrowding, owner occupation, and unemployment. Higher Townsend scores equate to higher levels of area-based socioeconomic deprivation.
Physical activity was self-reported and weighted for intensity: self-reported minutes of walking (×3.3), moderate exercise (×4.0), and vigorous exercise (×8.0; this is a common calculation21). These were then summated to create an overall physical activity score, which was then split into quintiles to simplify analysis.
Participants whose body mass index (BMI) was 40 or over were considered very severely obese as per WHO guidelines; we chose a cutoff of 40 rather than say 30 (moderately obese) because there is evidence of reverse causality where moderately high BMI can show a protective effect under some circumstances.22 (Note that final results were virtually identical when we used a BMI of 30 as a cutoff.)
In terms of smoking, we compared never vs current smokers. Frequency of alcohol intake was recorded as never, special occasions only, 1–3 times per month, 1–2 times per week, 3–4 times per week, daily/almost daily. Because our interest is in high vs low alcohol intake, we split this into a binary variable: participants who reported “daily or almost daily” (i.e., high) vs “1–3 times a month”; “special occasions only” and “never” (i.e., low). Participants were asked if there was a reason they had stopped drinking, for example, due to doctor's advice, health precaution: participants who reported this were removed from analysis, to help reduce confounding where low alcohol intake was due to poor health.
Genetic data
UK Biobank genotyping was conducted by Affymetrix using a bespoke BiLEVE Axiom array for ∼50,000 participants and the remaining ∼450,000 on the Affymetrix UK Biobank Axiom array. All genetic data were quality controlled by UK Biobank as described in the protocol paper.23 The APOE ε genotype is directly genotyped. Further information on the genotyping process is available (ukbiobank.ac.uk/scientists-3/genetic-data), including detailed technical documentation (biobank.ctsu.ox.ac.uk/crystal/docs/genotyping_sample_workflow.pdf). The 2 APOE ε single nucleotide polymorphisms—rs7412 and rs429358—were both in Hardy-Weinberg equilibrium (p > 0.05) assessed with PLINK V1.90.24
Standard protocol approvals, registrations, and patient consents
This secondary data analysis study was conducted under generic approval from the NHS National Research Ethics Service (approval letter dated 17 June 2011, ref 11/NW/0382). Written informed consent was obtained from all participants in the study (consent for research, by UK Biobank).
Data availability statement
UK Biobank is an open access resource available to verified researchers upon application (ukbiobank.ac.uk/). Analysis syntax is available upon request.
Statistical analysis
We used 2 models: partially adjusted and fully adjusted. The partially adjusted model was statistically corrected for the potential confounders of age, sex, genotypic array, assessment center, and 8 genetic principal components (to correct for potential stratification). The fully adjusted model was additionally corrected for Townsend deprivation scores, self-reported diabetes, CHD, hypertension, and university/college degree (yes vs no).1 We report descriptive statistics according to EQUATOR guidelines. The dependent variables in the linear regression were the cognitive scores for reasoning, log RT, log TMT-A and -B, and DSST scores.
We first tested for associations between APOE ε4 and lifestyle factors on cognitive abilities, using linear regression and reporting standardized β (i.e., on a per-SD scale of effect). We then tested for 2-way interactions between APOE ε4 genotype with male vs female sex, and ε4 with lifestyle factors. Finally, we tested for additional 3-way interactions (APOE, sex, lifestyle). TMT and reaction time scores were log-transformed due to a positive skew. We removed outliers above 3.30 SDs from the mean (<0.1%). We corrected for multiple testing using the False Discovery Rate (FDR).25,26 Power calculations were performed using G*Power 3.27 Stata V.14 was used for statistical analyses. For additional comparison with previous meta-analyses, we have provided Cohen d effect size estimates for unadjusted APOE ε4/cognitive associations (supplementary material, doi.org/10.5061/dryad.k66n2g2).
Results
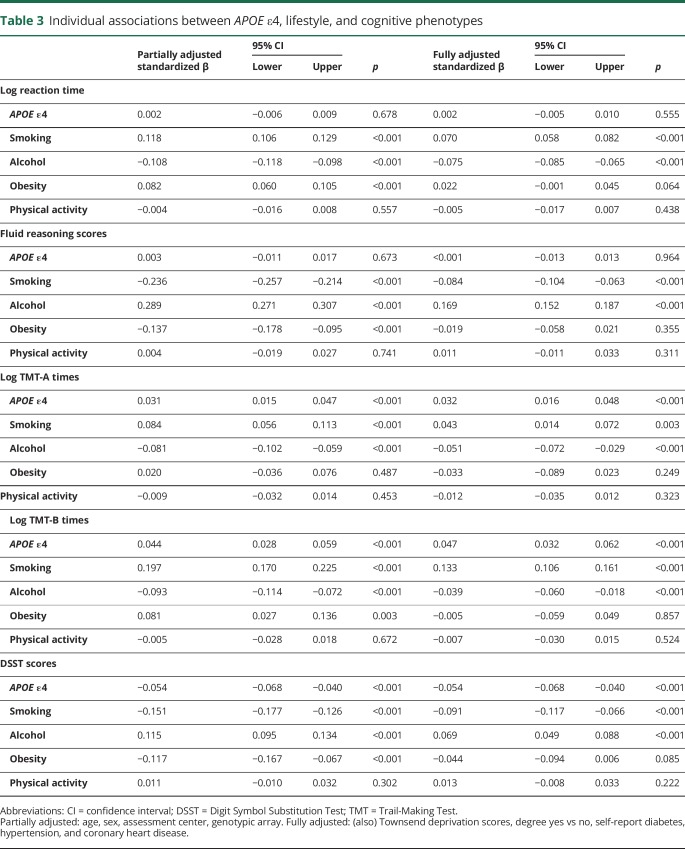
There were 487,377 participants with APOE ε genotype data. We excluded participants with non-white British ancestry, self-report vs genetic sex mismatch, putative sex chromosomal aneuploidy, excess heterozygosity, and missingness rate >0.1. This left n = 408,228. We removed participants who reported a neurologic condition (∼5%; see reference 14), the inclusion of which could drive type 1 errors due to skewed results (results were unchanged when we included these participants). This left 389,778 participants. Finally, we accounted for relatedness between participants by removing one random participant in cases where 2 individuals were first cousins or closer. This left 326,535 participants for whom genotype frequencies of APOE were ε2/ε2 n = 2,133 (1%), ε2/ε3 n = 40,460 (12%), ε2/ε4 = 8,348 (3%), ε3/ε3 = 189,728 (58.0%), ε3/ε4 n = 77,963 (24%), and ε4/ε4 n = 7,923 (2%). Descriptive statistics for cognitive scores and cardiometabolic conditions are shown in tables 1 and 2, and demographic factors are shown in table e-1 (doi.org/10.5061/dryad.k66n2g2).
Table 1.
Demographic descriptive statistics
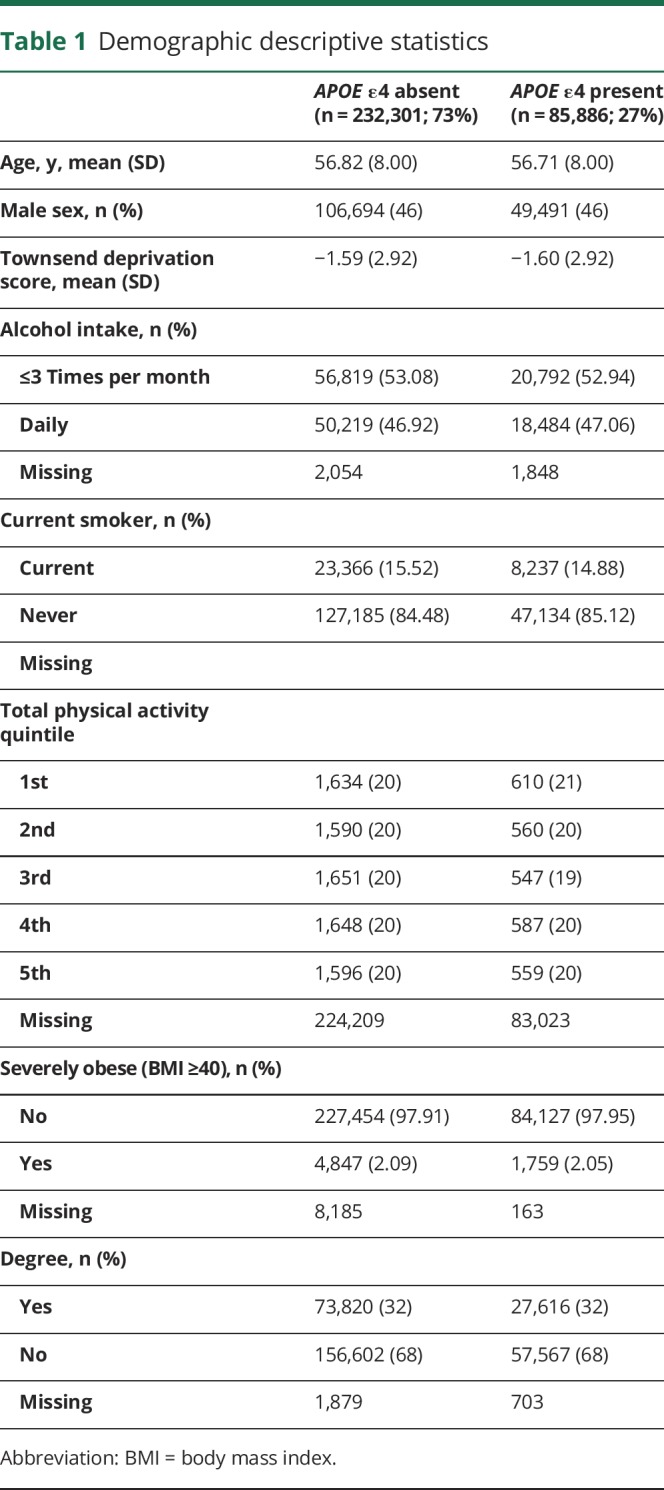
Table 2.
Cognitive score descriptive statistics
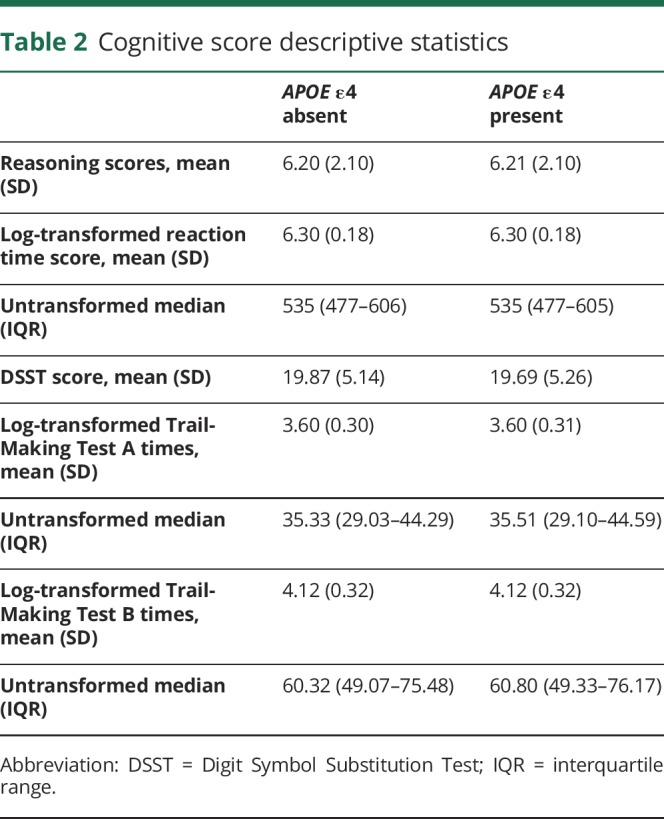
The mean age at baseline was 56.79 years (SD 8.00), and 150,071 (46%) participants were male. The mean age at time of completing the Internet tests was 61.8 years (SD 7.60). Using an APOE ε4 present vs absent model excluding ε2/ε4 (protective/risk alleles) genotype carriers results in sample sizes per group of ε4+ n = 85,886 (ε3/ε4; ε4ε4) vs ε4− n = 232,301 (ε2/ε2; ε2/ε3; ε3/ε3), total n = 318,187. In terms of cognitive data, reasoning data were available in n = 105,913, reaction time in n = 324,725, TMT-A (processing speed) in n = 70,988 and TMT-B (speed plus executive function) in n = 71,055, with DSST (executive function) in n = 79,840. All significant phenotypic/genetic associations with cognitive abilities reported hereafter remained significant after correction for type 1 error.
A power calculation showed that based on a Cohen d of 0.1 (a small effect size being 0.2) and group difference ratio of 2:1 (based arbitrarily on never vs current smoker ratio), 95% power to detect an effect would be achieved at n = 4,872, suggesting the current analyses have generally good power.
APOE ε4 and lifestyle associations with cognitive abilities
Table 3 shows standardized β associations between APOE ε4 genotype, lifestyle factors, and cognitive abilities: there were significant associations between ε4 genotype and worse log TMT-A times (fully adjusted model standardized β 0.032, 95% confidence interval [CI] 0.016–0.048, p < 0.001), TMT-B times (fully adjusted standardized β 0.047, 95% CI 0.032–0.062, p < 0.001), and DSST scores (fully adjusted standardized β −0.054, 95% CI −0.068 to −0.040, p < 0.001).
Table 3.
Individual associations between APOE ε4, lifestyle, and cognitive phenotypes
Unadjusted APOE ε4/cognitive score associations were of very small magnitude (i.e., under 0.2) for each of log RT (Cohen d = 0.003), reasoning (d = −0.003), log TMT-A (d = −0.014), log TMT-B (−0.023), and DSST coding (d = 0.035). Effect sizes were similar for untransformed RT and TMT-A/B values.
In terms of lifestyle factors, there were significant associations for smoking with reasoning, TMT-A and -B times, and DSST scores (all p < 0.001; table 1). There were significant associations for alcohol intake and obesity, but the sign of these associations changed for alcohol and obesity, where they appeared protective in the fully adjusted models for various tests. Physical activity did not significantly associate with any cognitive outcomes. When all analyses were corrected for type 1 error with FDR, all significant associations remained statistically significant (FDR-adjusted p values all < 0.05).
Two-way interactions: APOE ε4 and sex; APOE ε4 and lifestyle
We tested for APOE ε4 by sex interactions, with the results shown in table e-2 (doi.org/10.5061/dryad.k66n2g2). There were 2 significant interactions: for log RT (fully-adjusted model p = 0.045) and fluid reasoning (p = 0.034). Stratifying by sex using the fully adjusted models showed that the ε4 effect was stronger in male vs female participants for log RT (p = 0.068 vs 0.375, respectively) although still nonsignificant and not appreciably different for fluid reasoning scores (p = 0.155 vs 0.136). For DSST, there was a significant interaction between ε4 and obesity (final model p value < 0.001). Stratified, this appeared to be due to a significantly deleterious effect of ε4 genotype in nonobese participants (fully adjusted standardized β −0.058, 95% CI −0.072 to −0.044, p < 0.001), but protective in obese participants (fully adjusted standardized β 0.176, 95% CI 0.058–0.295, p = 0.004). All other tested 2-way interactions were not significant (p > 0.05).
Three-way interactions: APOE ε4, sex, and lifestyle
We tested for significant APOE ε4/sex/lifestyle interactions, with the results shown in table e-3 (doi.org/10.5061/dryad.k66n2g2). All interactions were nonsignificant except one. The significant interaction was for ε4 presence, sex, and high alcohol intake (i.e., daily or almost daily) vs not on reasoning scores (p = 0.020). Figure e-1 (doi.org/10.5061/dryad.k66n2g2) shows that the interaction was principally driven by male participants having a larger association between high alcohol intake and better reasoning (compared with female participants). While visually an ε4 effect becomes slightly larger in the context of high alcohol intake, pairwise comparisons did not show this to be statistically significant (p > 0.05). When all analyses were corrected for type 1 error with FDR, all significant interactions attenuated to nonsignificance (FDR-adjusted p values all >0.05). The total model adjusted r2 values ranged from 0.02 to 0.22 (i.e., 2%–22% of total variance explained).
Additional analyses
As post hoc analyses, we additionally repeated all tests for collated (potentially protective) APOE ε2/ε2 plus ε2/ε3 genotypes, vs neutral ε3/ε3. We also repeated the analyses with log-transformed (+1) pairs-matching error scores as an outcome. There were no significant associations or interactions once adjusted for FDR (all q values p > 0.100; results are available upon request).
It is possible that ε4 genotype and lifestyle are not independent. Logistic regressions showed that participants who possessed the ε4 allele were significantly less likely to smoke (odds ratio [OR] 0.95, 95% CI 0.93–0.98, p < 0.001) and more likely to have a degree (OR 1.02, 95% CI 1.00–1.03, p = 0.043), although the effect sizes were small, and carriers showed no differences in other lifestyle factors (see table e-4, doi.org/10.5061/dryad.k66n2g2, which shows all intercorrelations).
The protective effect of alcohol intake on cognitive ability is counterintuitive, having removed people who reported stopping due to ill health. Descriptive statistics of alcohol intake by APOE ε4 genotype status are shown in table e-5 (doi.org/10.5061/dryad.k66n2g2).
Discussion
This study hypothesized that based on previous studies in smaller cohorts, together with biological rationale, risk factors for worse cognitive ability such as smoking history, (high) alcohol intake, obesity, and lower physical activity would interact with APOE ε4 genotype, such that each risk factor's association with worse cognitive scores would be larger in ε4 carriers (vs noncarriers). We also investigated the moderating role of sex.28 We found that associations between APOE ε4 and cognitive scores were of relatively small effect size, and only suggestive interactions with sex, where ε4 men scored worse than women (which did not survive correction for multiple testing and in any case the within-sex ε4 effects were not nominally significant). We also found some small, counterintuitive suggestive results; for example, that severe obesity and daily drinking could be protective. These findings could reflect test imprecision, the generally preserved and healthy sample (i.e., selection or attrition biases), underestimation of ε4's true effect (due to attrition), or that previous studies perhaps overstated the true effect. Our findings generally support a direct route of APOE ε4 genotype to cognitive decline rather than increasing vulnerability to other factors.
In this study we report negative associations between smoking and worse cognitive ability, which fits the established literature,29 although surprisingly protective associations from high alcohol intake (i.e., daily) and obesity, defined here as BMI of 40 and above (severely obese), even after adjusting for prevalent diseases and accounting as much as possible for people whose alcohol intake had significantly changed in recent years due to ill health (i.e., factors that might cause reverse causality). This is more likely to reflect selection or collider bias in some way30; for example, where the participants who drink more/are highly obese and respond positively to the invitation for assessment are selected for,31 rather than the association being causal. This is also the most likely explanation for ε4 carriers having better scores (vs noncarriers) in the context of severe obesity in this study. In any case, the interactions were null after correction for type 1 error with FDR. There was no association from weighted physical activity, although the sample size for that variable was much smaller than others. There were significant associations between APOE ε4 genotype and worse TMT-A, TMT-B, and DSST scores, which fits previous literature that ε4 genotype is deleterious for processing speed and executive function.32
There were mostly no statistically significant interactions between lifestyle factors and APOE ε4 genotype. The ε4/cognitive associations were of small magnitude, compared to previous meta-analyses.33 Power analysis estimates showed that we had relatively good power to detect an association, although it is still possible that the lack of association reflects a lack of power. Alternative interpretations include that the UK Biobank participants have perhaps not deteriorated markedly with age or are in generally good health, or are slightly too young (mean age 56 at baseline) to show significant effects of APOE ε4 genotype, which can show a larger association with cognitive function with increasing age34 or longitudinally.32 Further to this, there may be sex effects that vary by age window: for example, Neu et al.35 found that APOE ε3/ε4 genotype was associated with earlier age at onset of Alzheimer disease (AD) (vs men; total n = 57,979), and Hohman et al.36 reported significant interaction between ε4 presence (vs absence) and female (vs male) sex on higher total CSF total and phosphorylated tau (a neuropathologic marker of AD). Additional interactions that we did not assess are also possible, for example, between APOE ε4, sex, and deprivation level, and this will be an interesting area of future research.
It is possible that the lack of interaction reflects a degree of selection bias where the sample includes healthier carriers of the ε4 genotype (generally reported as deleterious), and its effect in this cohort is therefore underestimated to an extent.
Our results slightly contrast with our previous findings in around 110,000 UK Biobank participants, where we reported a significant deleterious interaction between ε4 genotype and reasoning scores (p < 0.001); however, this (and all other tests) did not survive correction for additional covariates; for example, depression, Townsend scores, and cardiometabolic conditions in that study.
We have reported previously on potential limitations of the novel baseline tests: namely that the reasoning test includes some “crystallized” (i.e., accumulated knowledge) items that are not strictly reasoning, and the reliabilities are poorer across time compared with more standard, validated cognitive tests.14 We did not report on UK Biobank memory scores because our previous analysis has shown that (1) the test was not reliable across time14 and (2) ε4 had no major association with scores in 110,000 anyway.1 The web-based tests are more akin to existing validated cognitive batteries, but their use over the Internet in this instance has not been characterized and there may be some inaccuracies due to Internet connection lag or computer problems in people's homes. It is possible that the interaction between ε4 genotype and lifestyle risk factors has been overstated due to publication bias, particularly given that many studies are small in terms of sample size.37 On the other hand, the large sample size used here may increase risk of statistically significant findings that are of such small magnitude as to not be practically or clinically significant.
The UK Biobank does not have a metric of premorbid, lifetime cognitive ability in its participants. This could be an important limitation, where brighter young adults are less likely to engage in unhealthy behaviors, or in midlife, people with better cognitive ability may be better able to manage their health care and take medications reliably.38
Genetic modification of phenotypic risk factors on cognitive ability has enormous potential implication for prevention of cognitive impairment in an aging population. Future research may seek to investigate this question in brain imaging phenotypes (available in UK Biobank, although in smaller numbers), as these factors are less downstream of the effects of genetic variation compared with cognitive scores, which can be affected by state-dependent factors like stress or anxiety.39
This study aimed to test for interactions between APOE ε4, lifestyle, and sex on cognitive abilities. We found suggestive interaction test results where men were more vulnerable to ε4 genotype (in terms of cognition). Caveats to this were that the effect sizes were small, and there may be biases at play (e.g., where ε4's effects are underestimated in the data). Our results therefore provide less support for the idea that ε4 genotype increases vulnerability to the negative effects of lifestyle risk factors, but rather support a primarily outright association between APOE ε4 genotype and worse cognitive ability.
Acknowledgment
This research has been conducted using the UK Biobank resource (project code 17689). The authors thank the UK Biobank participants and Dr. Breda Cullen for devising exclusion criteria.
Glossary
- AD
Alzheimer disease
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- DSST
Digit Symbol Substitution Test
- FDR
False Discovery Rate
- RT
reaction time
- TMT
Trail-Making Test
Author contributions
Study concept or design: D.M.L. Drafting/revising the manuscript for content: D.M.L., C.C.-M., L.M.L., N.G., D.F.M., R.J.S., J.W., J.M.G., NS., J.C., D.J.S., J.P.P. Analysis or interpretation of data: D.M.L., C.C.-M., L.M.L., N.G., D.F.M., R.J.S., J.W., J.M.G., N.S., J.C., D.J.S., J.P.P.
Study funding
UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. The funders had no role in study design, data collection or management, analyses or interpretation of the data, or preparation, review or approval of the manuscript. Supported by MRC Mental Health Data Pathfinder Award (reference MC_PC_17,217).
Disclosure
D. Lyall, C. Graham, N. Graham, D. Mackay, R. Strawbridge, J. Ward, and J. Gill report no disclosures relevant to the manuscript. N. Sattar has consulted for Amgen, Inc., Sanofi, AstraZeneca, and Eli Lilly, and has sat on the Medical UK Biobank Scientific Advisory Board. J. Cavanagh is funded by the Sackler Trust, Wellcome Trust, and Medical Research Council, holds a Wellcome Trust strategic award, and has an industrial–academic collaboration with Janssen & Janssen, GlaxoSmithKline, and Lundbeck. D. Smith is partially funded by the Lister institute. J. Pell has received funding from the Medical Research Council and Chief Scientist Office and has sat on the Medical Research Council Strategy Board and UK Biobank Scientific Advisory Board. Go to Neurology.org/N for full disclosures.
References
- 1.Lyall DM, Ward J, Ritchie SJ, et al. Alzheimer disease genetic risk factor APOE ε4 and cognitive abilities in 111,739 UK Biobank participants. Age Ageing 2016;45:511–577. [DOI] [PubMed] [Google Scholar]
- 2.Lyons MJ, Genderson M, Grant MD, et al. Gene-environment interaction of ApoE genotype and combat exposure on PTSD. Am J Med Genet B Neuropsychiatr Genet 2013;162:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Bao Y, He S, et al. The association between apolipoprotein E and functional outcome after traumatic brain injury. Medicine 2015;94:e2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol 2012;69:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 2017;7:e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies G, Harris SE, Reynolds CA, et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry 2014;19:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser VA, Pike CJ. Obesity and sex interact in the regulation of Alzheimer's disease. Neurosci Biobehav Rev 2016;67:102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med 2008;12:2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med 2012;2:a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez FS, Schroeter ML, Arélin K, et al. APOE ε4-genotype and lifestyle interaction on cognitive performance: results of the LIFE-Adult-Study. Heal 2018;37:194–205. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JPA, Munafò MR, Fusar-Poli P, Nosek BA, David SP. Publication and other reporting biases in cognitive sciences: detection, prevalence, and prevention. Trends Cogn Sci 2014;18:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyall DM, Cullen B, Allerhand M, et al. Cognitive test scores in UK Biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One 2016;11:e0156366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies G, Marioni REE, Liewald DCC, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N=112 151). Mol Psychiatry 2016;21:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. NIH Public Access 2011;39:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salthouse TA. What do adult age differences in the digit symbol substitution test reflect? J Gerontol Psychol Sci 1992;47:21–128. [DOI] [PubMed] [Google Scholar]
- 18.Fellows RP, Dahmen J, Cook D, Schmitter-Edgecombe M. Multicomponent analysis of a digital trail making test. Clin Neuropsychol 2017;31:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol 2006;21:623–643. [DOI] [PubMed] [Google Scholar]
- 20.Townsend P. Townsend deprivation index. National Database Primary Care Groups Trust 1998. [Google Scholar]
- 21.Siebert S, Lyall DM, Mackay DF, et al. Characteristics of rheumatoid arthritis and its association with major comorbid conditions: cross-sectional study of 502 649 UK Biobank participants. RMD Open 2016;2:e000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimer's Dement 2018;14:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ∼500,000 UK Biobank participants. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; Epub 2017 Jul 20: 166298.
- 24.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2011;2:278–282. [Google Scholar]
- 26.Benjamini Y, Hochberg Y, Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300. [Google Scholar]
- 27.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 28.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheimers Dement 2018;14:1171–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karama S, Ducharme S, Corley J, et al. Cigarette smoking and thinning of the brain’s cortex. Mol Psychiatry 2015;20:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day FR, Loh PR, Scott RA, Ong KK, Perry JR. A robust example of collider bias in a genetic association study. Am J Hum Genet 2016;98:392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millard LAC, Davies NM, Tilling K, Gaunt TR, Smith GD. Searching for the causal effects of body mass index in over 300 000 individuals, using Mendelian randomization. PLoS Genet 2019;15:e1007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyall DM, Harris SE, Bastin ME, et al. Are APOE ɛ genotype and TOMM40 poly-T repeat length associations with cognitive ageing mediated by brain white matter tract integrity? Transl Psychiatry 2014;4:ε449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging 2011;32:63–74. [DOI] [PubMed] [Google Scholar]
- 34.Schiepers OJG, Harris SE, Gow AJ, et al. APOE Ε4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry 2011;17:315–324. [DOI] [PubMed] [Google Scholar]
- 35.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease. JAMA Neurol 2017;74:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol 2018;75:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munafò MR, Stothart G, Flint J. Bias in genetic association studies and impact factor. Mol Psychiatry 2009;14:119–120. [DOI] [PubMed] [Google Scholar]
- 38.Calvin CM, Deary IJ, Fenton C, et al. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. Int J Epidemiol 2011;40:626–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci 2010;11:201–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
UK Biobank is an open access resource available to verified researchers upon application (ukbiobank.ac.uk/). Analysis syntax is available upon request.