Extended Data Fig. 3 |. Expression, purification and characterization of soluble PCDH1 proteins.
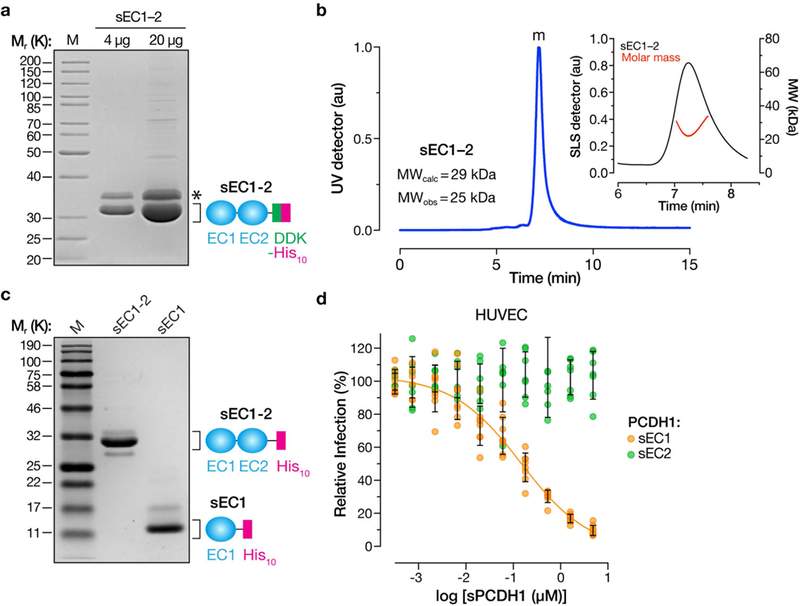
a, Purified sEC1–2 bearing C-terminal Flag and decahistidine (His10) epitope tags was resolved on an SDS–polyacrylamide gel and visualized by Coomassie blue staining. Asterisk, minor component of unknown origin. Mr, relative molecular weight (K denotes × 1,000); M, monomer peak. The experiment was performed three times with similar results. b, SEC-MALS analysis of purified sEC1–2 from a. Absorbance (arbitrary units, au) was monitored at 280 nm; m, monomer peak. Calculated (MWcalc) and observed (MWobs) molecular-weight estimates from MALS are shown in the inset. The experiment was performed twice with similar results. c, Generation of purified sEC1, comprising the first extracellular cadherin domain of PCDH1, and bearing a C-terminal His10 epitope tag. sEC1–2 is shown for comparison. The experiment was performed three times with similar results. d, Capacity of sEC1 and sEC2 to block hantavirus glycoprotein-dependent entry. rVSVs bearing ANDV Gn/Gc were preincubated with sEC1 or sEC2 (0–5 μM, in serial threefold dilutions), and then allowed to infect HUVECs at a MOI of 0.2 IU per cell. Cells were scored for infection at 9 hpi. One hundred per cent relative infection corresponds to 10–20% infected cells. Averages ± s.d.: three experiments, n = 8 for sEC1; n = 7 for sEC2.
