Abstract
Problem:
The immunophenotype of B cells at the maternal-fetal interface (decidua) in labor at term and preterm labor is poorly understood.
Method of study:
Decidual tissues were obtained from women with preterm or term labor and from non-labor gestational age-matched controls. Immunophenotyping of decidual B cells was performed using multi-color flow cytometry.
Results:
1) In the absence of acute or chronic chorioamnionitis, total B cells were more abundant in the decidua parietalis of women who delivered preterm than those who delivered at term, regardless of the presence of labor; 2) decidual transitional and naïve B cells were the most abundant B-cell subsets; 3) decidual B1 B cells were increased in women with labor at term or preterm labor and chronic chorioamnionitis compared to those without this placental lesion; 4) decidual transitional B cells were reduced in women with preterm labor compared to those without labor; 5) naïve, class-switched, and non-class-switched B cells in the decidual tissues underwent mild alterations with the process of preterm labor and/or placental inflammation; 6) decidual plasmablasts seemed to increase in women with labor at term or preterm labor with chronic chorioamnionitis; and 7) decidual B cells expressed high levels of interleukin (IL)-12, IL-6 and/or IL-35.
Conclusions:
Total B cells are not increased with the presence of preterm or term labor; yet, specific subsets (B1 and plasmablasts) undergo alterations in women with chronic chorioamnionitis. Therefore, B cells are solely implicated in the pathological process of preterm labor in a subset of women with chronic inflammation of the placenta. These findings provide insight into the immunology of the maternal-fetal interface in preterm and term labor.
Keywords: B1 B cells, chronic chorioamnionitis, funisitis, memory B cells, naïve B cells, placental inflammation, plasmablasts, pregnancy, transitional B cells
INTRODUCTION
Preterm labor, which commonly precedes preterm birth 1, 2, is a syndrome involving multiple pathological processes 3, 4. Among the known mechanisms, pathological inflammation is the best-characterized causal link to preterm labor and birth 5–14. To date, the most studied causes of pathological inflammation leading to preterm labor have been 1) intra-amniotic infection/inflammation resulting from microbial invasion of the amniotic cavity 5, 7, 8, 15–33, and 2) intra-amniotic inflammation without detectable microorganisms (i.e. sterile intra-amniotic inflammation) identified by using both molecular and conventional microbiological techniques 32, 34–38, proposed to be due to endogenous danger signals, or alarmins 39–49. Most research concerning inflammation-induced preterm labor has therefore focused on the innate limb of immunity 50–73. Yet, several studies reported strong evidence that T cells, the primary cellular component of the adaptive immune system, are present at the maternal-fetal interface 74–89. More recently, we provided evidence indicating that T cells are also implicated in the mechanisms that lead to labor at term 84, 85 and spontaneous preterm labor 90–97. However, B cells, the main humoral component of adaptive immunity, have been less investigated.
B cells were first described in the placental bed of women early in gestation 98, which was confirmed by later studies 99, 100. During early pregnancy, B cells are implicated in the mechanisms of maternal-fetal tolerance 101–115. Decidual B cells modestly increased between 27 and 33 weeks of gestation followed by a slight decline at term 100. In the absence of labor at term, multiple studies reported that B cells are present at the human maternal-fetal interface (i.e. decidua basalis and decidua parietalis) 76, 77, 99, 116, 117. Moreover, B cells seem to be increased in the decidua basalis 77, but not in the decidua parietalis 77, 116, during the physiological process of labor at term. A recent study provided evidence indicating a role for B cells in the pathogenesis of preterm labor: the results showed increased proportions of B cells in the decidua parietalis of women who underwent spontaneous preterm labor compared to those with labor at term 118. However, this study did not include gestational age-matched controls, allowing for further investigation of the B-cell compartment at the human maternal-fetal interface in both labor at term and preterm labor.
In the current study, we performed immunophenotyping of the decidua basalis and decidua parietalis of women who underwent the physiological process of labor at term or the syndrome of preterm labor leading to preterm birth. Decidual tissues from gestational age-matched controls were also included. In addition, the B-cell subsets in acute and chronic maternal inflammatory lesions of the placenta were compared. Lastly, the production of cytokines by decidual B cells was determined.
MATERIALS AND METHODS
Human subjects, clinical specimens, and definitions
Samples of the human placental basal plate (maternal side of the placenta, decidua basalis) and chorioamniotic membranes (amnion, chorion, and decidua parietalis) were collected from patients within 30 minutes after delivery at Hutzel Women’s Hospital in the Detroit Medical Center, Detroit, MI, USA, in partnership with Wayne State University School of Medicine and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), Detroit, MI, USA. The collection and utilization of biological materials for research purposes were approved by the Institutional Review Boards of Wayne State University and NICHD. All participating women provided written informed consent prior to the collection of samples.
The study groups included women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). Preterm birth was defined as delivery before 37 weeks of gestation. Labor was defined by the presence of regular uterine contractions at a frequency of at least 2 contractions every 10 minutes with cervical changes resulting in delivery. The TIL and PTL study groups were subdivided based on the presence of acute histologic chorioamnionitis (ACA) and chronic histologic chorioamnionitis (CCA) (see “Placental histopathological examination” section for diagnostic criteria). Patients with neonates having congenital or chromosomal abnormalities were excluded from this study. The clinical and demographic characteristics of the study population are shown in Tables 1 and 2. Both the decidua basalis and decidua parietalis were collected from most patients; however, the decidua basalis was not available in a few cases. Therefore, Table 1 describes patients from which the decidua basalis was available, and Table 2 describes patients from which the decidua parietalis was available for experiments.
Table 1.
Clinical and demographic characteristics of the patient population used to perform immunophenotyping of the decidua basalis
| Term without labor (n=15) |
Term labor (n=14) |
Term labor with ACA (n=10) |
Term labor with CCA (n=13) |
Preterm without labor (n=4) |
Preterm labor (n=8) |
Preterm labor with ACA (n=4) |
Preterm labor with CCA (n=14) |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 24 (21–29) | 25 (21.3–30.3) | 23.5 (22–25.8) | 24 (22–29) | 30.5 (28–31) | 26 (20.5–29) | 34 (29.5–37) | 24.5 (21–29.8) | 0.5 |
| Body mass index (kg/m2; median [IQR])a | 31.8 (29.5–34.8) | 30.2 (25.3–36.8) | 29 (26.6–33.2) | 31.3 (26.6–37.3) | 34.2 (31.8–37.1) | 30.2 (21.7–41.1)c | 28.3 (25.2–34.7) | 24.3 (21.6–38.2) | 0.7 |
| Primiparityb | 13.3% (2/15) | 28.6% (4/14) | 40% (4/10) | 23.1% (3/13) | 0% (0/4) | 12.5% (1/8) | 0% (0/4) | 14.3% (2/14) | 0.6 |
| Raceb | 0.6 | ||||||||
| African-American | 73.3% (11/15) | 78.6% (11/14) | 90% (9/10) | 92.3% (12/13) | 75% (3/4) | 62.5%(5/8) | 75% (3/4) | 78.6% (11/14) | |
| Caucasian | 13.3%(2/15) | 21.4% (3/14) | 0% (0/10) | 7.7% (1/13) | 25% (1/4) | 25% (2/8) | 25% (1/4) | 14.3% (2/14) | |
| Asian | 0% (0/15) | 0% (0/14) | 10% (1/10) | 0% (0/13) | 0% (0/4) | 0% (0/8) | 0% (0/4) | 0% (0/14) | |
| Other | 13.3% (2/15) | 0% (0/14) | 0% (0/10) | 0% (0/13) | 0% (0/4) | 12.5% (1/8) | 0% (0/4) | 7.1% (1/14) | |
| Cesarean section | 100% (15/15) | 14.3% (2/14) | 20% (2/10) | 7.7% (1/13) | 100% (4/4) | 37.5% (3/8) | 25% (1/4) | 21.4% (3/14) | <0.001 |
| Gestational age at delivery (weeks; median [IQR])a | 39.1 (39–39.4) | 39 (38.2–40.2) | 39.4 (38.4–39.8) | 39.6 (38.9–40.1) | 34.7 (33.1–36.1) | 34.4 (31–35.5) | 34 (32.8–34.7) | 34.9 (33.3–36.3) | <0.001 |
| Birthweight (grams)a | 3225 (2930–3482.5) | 3515 (3121.3-3645) | 3392.5 (3348.8–3628.8) | 3460 (3155-3665) | 2025.5 (1392–2798.8) | 1932.5 (1401.5–3233.8) | 2130 (1487.5–2776.3) | 2240 (1887.5–2322.5) | <0.001 |
Data are given as median (interquartile range, IQR) and percentage (n/N). ACA = acute chorioamnionitis; CCA = chronic chorioamnionitis (please see Methods for definitions).
Kruskal-Wallis test.
Fisher’s exact test.
One missing data.
Table 2.
Clinical and demographic characteristics of the patient population used to perform immunophenotyping of the decidua parietalis
| Term without labor (n=16) |
Term labor (n=14) |
Term labor with ACA (n=10) |
Term labor with CCA (n=14) |
Preterm without labor (n=4) |
Preterm labor (n=9) |
Preterm labor with ACA (n=4) |
Preterm labor
with CCA (n=14) |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 24 (21–28.8) | 25 (21.3–30.3) | 23.5 (22–25.8) | 24 (22–29) | 30.5 (28–31) | 28 (21–32) | 34 (29.5–37) | 24.5 (21–29.8) | 0.5 |
| Body mass index (kg/m2; median [IQR])a | 31.5 (29.5–34.4)c | 30.2 (25.3–36.8) | 29 (26.6–33.2) | 32.8 (26.7–37.1) | 34.2 (31.8–37.1) | 32.9 (21.9–40)c | 28.3 (25.2–34.7) | 24.3 (21.6–38.2) | 0.7 |
| Primiparityb | 12.5% (2/16) | 28.6% (4/14) | 40% (4/10) | 21.4%(3/14) | 0% (0/4) | 11.1% (1/9) | 0%(0/4) | 14.3% (2/14) | 0.6 |
| Raceb | 0.5 | ||||||||
| African-American | 75% (12/16) | 78.6% (11/14) | 90% (9/10) | 92.9% (13/14) | 75% (3/4) | 55.6% (5/9) | 75% (3/4) | 78.6% (11/14) | |
| Caucasian | 18.8% (3/16) | 21.4% (3/14) | 0% (0/10) | 7.1% (1/14) | 25% (1/4) | 33.3% (3/9) | 25% (1/4) | 14.3% (2/14) | |
| Asian | 0% (0/16) | 0% (0/14) | 10% (1/10) | 0% (0/14) | 0% (0/4) | 0% (0/9) | 0% (0/4) | 0% (0/14) | |
| Other | 6.2% (1/16) | 0% (0/14) | 0% (0/10) | 0% (0/14) | 0% (0/4) | 11.1% (1/9) | 0% (0/4) | 7.1% (1/14) | |
| Cesarean section | 100% (16/16) | 14.3% (2/14) | 20% (2/10) | 7.1% (1/14) | 100% (4/4) | 33.3% (3/9) | 25% (1/4) | 21.4% (3/14) | <0.001 |
| Gestational age at delivery (weeks; median [IQR])a | 39.1 (39–39.2) | 39 (38.2–40.2) | 39.4 (38.4–39.8) | 39.7 (38.9–40.1) | 34.7 (33.1–36.1) | 34.7 (31.3–35.9) | 34 (32.8–34.7) | 34.9 (33.3–36.3) | <0.001 |
| Birthweight (grams)a | 3222.5 (2940–3456.3) | 3515 (3121.3–3645) | 3392.5 (3348.8–3628.8) | 3510 (3206.3–3706.3) | 2025.5 (1392–2798.8) | 2160 (1435-3155) | 2130 (1487.5–2776.3) | 2240 (1887.5–2322.5) | <0.001 |
Data are given as median (interquartile range, IQR) and percentage (n/N). ACA = acute chorioamnionitis; CCA = chronic chorioamnionitis (please see Methods for definitions).
Kruskal-Wallis test.
Fisher’s exact test.
One missing data.
Placental histopathological examination
Placentas were examined histologically by a perinatal pathologist blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols. Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; other sections were taken randomly from the placental disc. Acute and chronic inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading 94, 119–122. Maternal acute placental inflammation was defined by the infiltration of neutrophils into the chorion and amnion, termed acute histologic chorioamnionitis (ACA) 119, 121. Maternal chronic histologic chorioamnionitis (CCA) was defined as lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue 90, 94.
Decidual leukocyte isolation
Decidual leukocytes were isolated from the decidual tissue of patients from each study group as previously described 123. Briefly, the decidua basalis was collected from the basal plate of the placenta, and the decidua parietalis was separated from the chorioamniotic membranes. The decidual tissues were homogenized in StemPro Accutase Cell Dissociation Reagent (Life Technologies, Grand Island, NY, USA) using a gentleMACS Dissociator (Miltenyi Biotec, San Diego, CA, USA). Homogenized tissues were incubated in Accutase for 45 min at 37°C with gentle agitation. After incubation, tissues were washed in 1X phosphate‐buffered saline (PBS; Life Technologies) and filtered through a 100‐μm cell strainer (Fisher Scientific, Durham, NC, USA). The resulting cell suspensions were centrifuged at 300 × g for 10 min at 4°C. The decidual mononuclear cells were then separated using a density gradient (Ficoll‐Paque Plus; GE Healthcare Biosciences, Piscataway, NJ, USA) in accordance with the manufacturer’s instructions. The cells collected from the mononuclear layer of the density gradient were washed with 1X PBS and immediately used for immunophenotyping.
Immunophenotyping of decidual B cells
Mononuclear cell suspensions from decidual tissues were stained with the LIVE/DEAD Fixable Yellow Dead Cell Stain Kit (ThermoFisher Scientific/Molecular Probes, Eugene, OR, USA) prior to immunophenotyping. Mononuclear cell suspensions were then washed with stain buffer (CAT#554656; BD Biosciences, San Jose, CA, USA) and incubated with 20 μL of human FcR Blocking Reagent (CAT#130‐059‐901; Miltenyi Biotec) in 80 μL of stain buffer for 10 min at 4°C. The cells were incubated with extracellular fluorochrome‐conjugated anti‐human monoclonal antibodies for 30 min at 4°C in the dark (Supplementary Table 1). Stained cells were washed and resuspended in 0.5 mL of FACS staining buffer and acquired using an LSRII flow cytometer and FACSDiva 6.0 software (BD Biosciences). The absolute number of cells was determined using CountBright absolute counting beads (Life Technologies, Molecular Probes). The analysis and figures were performed using FlowJo software version 10 (FlowJo, LLC, Ashland, OR, USA). Lymphocytes were gated using forward scatter (FSC) verse side scatter (SSC). B cells were gated as CD19+CD3− cells within the lymphocytic and viability gates (Figure 1B). The cell surface markers (Supplementary Table 1) used to identify the different B-cell subsets were determined by an extensive literature search (Table 3). Cytokine expression by decidual B cells was also performed using specific monoclonal antibodies directly after isolation from the tissue without stimulation (Supplementary Table 1).
Figure 1. Immunophenotyping of B cells in the decidua basalis and decidua parietalis.
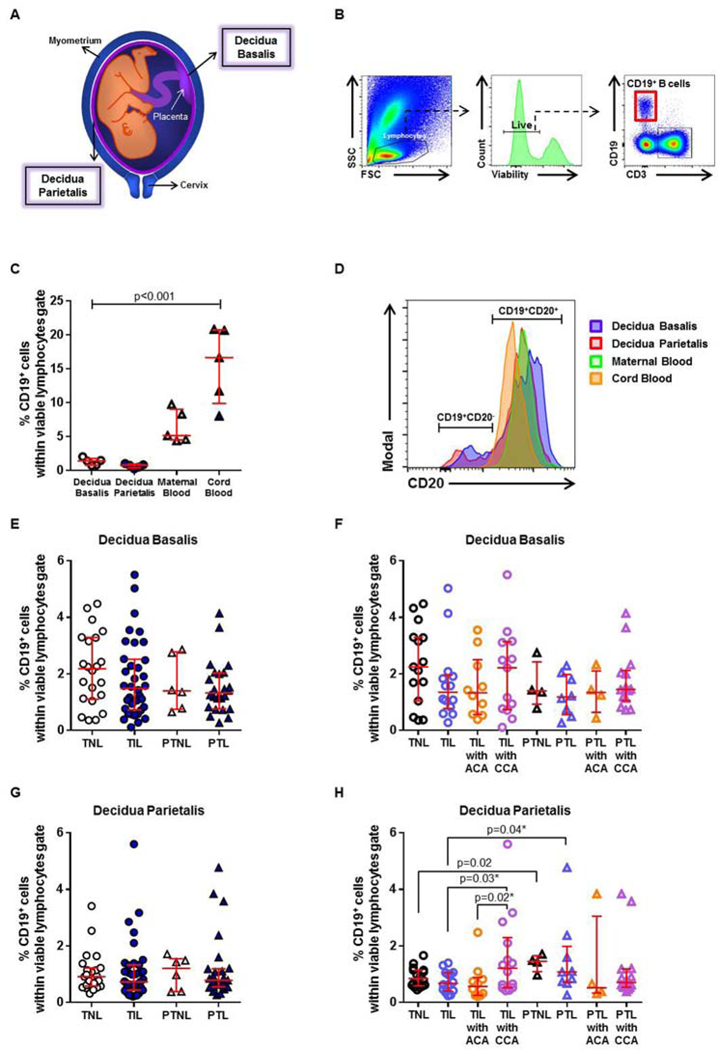
(A) Representation of the spatial localization of the decidua basalis and decidua parietalis. (B) Flow cytometry gating strategy used to identify B cells in the decidual tissues. Lymphocytes were gated using forward scatter (FSC) versus side scatter (SSC). B cells were gated as CD19+CD3− cells within the viability and lymphocytic gates. (C) The proportion of CD19+ B cells in samples of case-matched decidua basalis, decidua parietalis, maternal blood, and cord blood (n = 5). (D) Co-expression of CD20 by CD19+ B cells in the decidua basalis, decidua parietalis, maternal blood, and cord blood. The proportions of CD19+ B cells in the decidua basalis (E) or decidua parietalis (G) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of CD19+ B cells in the decidua basalis (F) or decidua parietalis (H) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Table 3.
Markers to identify B cells subsets
| B cell subsets | Immunophenotype | References |
|---|---|---|
| B1 B cells | CD19+CD27+CD43+ | Griffin DO et.al[1, 2], Torring C et.al[3], Inui M et.al[4] |
| B2 B cells | CD19+CD23+ CD27-CD43- |
Griffin DO et.al[1], Deng C et.al[5] |
| Transitional B cells | CD19+CD38hiCD24hi | Marie-Cardine A et.al[6], Ha Y et.al[7], Seifert M et.al[8], de Masson A et.al[9], Cherukuri A et.al[10], Heidt S et.al[11], Latorre I et.al[12], Tebbe B et.al[13], Luk F et.al[14], Demoersman J et.al[15], Li S et.al[16] |
| Naïve B cells | CD19+CD27-IgD+ | Guerreiro-Cacais A et.al[17], So N et.al[18], Heath E et.al[19], Cantaert T et.al[20], Toapanta F et.al[21], Jansen M et.al[22], Castaneda D et.al[23], Wu X et.al[24], Nakayama Y et.al[25] |
| Class-switched memory B cells | CD19+CD27+IgD- | Anolik J et.al[26], Tian C et.al[27], Ghannam A et.al[28], Palanichamy A et.al[29], Berkowska M et.al[30], Morbach H et.al[31], Wu Y et.al[32], So N et.al[18], Heath E et.al[19], Topanta F et.al[21], Labuda L et.al[33], Degauque N et.al[34], Zhang L et.al[35], Bagnara D et.al[36], Czarnowicki T et.al[37], Hayashi M et.al[38], Mensah F et.al[39], Woda M et.al[40], Castaneda D et.al[23], Martins C et.al[41] |
| Non class-switched memory B cells | CD19+CD27+IgD+ | Anolik J et.al[26], Tian C et.al[27], Palanichamy A et.al[29], Colonna-Romano G et.al[42], Jacobi A et.al[43], Wu Y et.al[32], So N et.al[18], Heath E et.al[19], Topanta F et.al[21], Weller S et.al[44], Labuda LA et.al[33], Clemente A et.al[45], Czarnowicki T et.al[37], Mensah F et.al[39], Castaneda D et.al[23], Martins C et.al[41], Corneth O et.al[46], Torigoe M et.al[47], Hu F, et.al[48] |
| Plasmablasts | CD19+CD20-CD38+CD24- | Morbach H et.al[49], Lin W et.al[50], Benett M et.al[51] |
References
Griffin, D.O., N.E. Holodick, and T.L. Rothstein, Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med, 2011. 208(1): p. 67–80.
Griffin, D.O. and T.L. Rothstein, A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med, 2011. 208(13): p. 2591–8.
Torring, C., et al., The B1-cell subpopulation is diminished in patients with relapsing-remitting multiple sclerosis. J Neuroimmunol, 2013. 262(1–2): p. 92–9.
Inui, M., et al., Human CD43+ B cells are closely related not only to memory B cells phenotypically but also to plasmablasts developmentally in healthy individuals. Int Immunol, 2015. 27(7): p. 345–55.
Deng, C., et al., The Imbalance of B-Lymphocyte Subsets in Subjects with Different Glucose Tolerance: Relationship with Metabolic Parameter and Disease Status. J Diabetes Res, 2017. 2017: p. 5052812.
Marie-Cardine, A., et al., Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol, 2008. 127(1): p. 14–25.
Ha, Y.J., et al., Characterization of phenotypically distinct B-cell subsets and receptor-stimulated mitogen-activated protein kinase activation in human cord blood B cells. J Leukoc Biol, 2008. 84(6): p. 1557–64.
Seifert, M., et al., Cellular origin and pathophysiology of chronic lymphocytic leukemia. J Exp Med, 2012. 209(12): p. 2183–98.
de Masson, A., H. Le Buanec, and J.D. Bouaziz, Purification and immunophenotypic characterization of human B cells with regulatory functions. Methods Mol Biol, 2014. 1190: p. 45–52.
Cherukuri, A., et al., Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol, 2014. 25(7): p. 1575–85.
Heidt, S., et al., B Cell Markers of Operational Tolerance Can Discriminate Acute Kidney Allograft Rejection From Stable Graft Function. Transplantation, 2015. 99(5): p. 1058–1064.
Latorre, I., et al., Calcineurin and mTOR inhibitors have opposing effects on regulatory T cells while reducing regulatory B cell populations in kidney transplant recipients. Transpl Immunol, 2016. 35: p. 1–6.
Tebbe, B., et al., Renal Transplant Recipients Treated with Calcineurin-Inhibitors Lack Circulating Immature Transitional CD19+CD24hiCD38hi Regulatory B-Lymphocytes. PLoS One, 2016. 11(4): p. e0153170.
Luk, F., et al., Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front Immunol, 2017. 8: p. 1042.
Demoersman, J., et al., B cell subset distribution is altered in patients with severe periodontitis. PLoS One, 2018. 13(2): p. e0192986.
Li, S., et al., Marked elevation of circulating CD19(+)CD38(hi)CD24(hi) transitional B cells give protection against neonatal sepsis. Pediatr Neonatol, 2018. 59(3): p. 296–304.
Guerreiro-Cacais, A.O., J. Levitskaya, and V. Levitsky, B cell receptor triggering sensitizes human B cells to TRAIL-induced apoptosis. J Leukoc Biol, 2010. 88(5): p. 937–45.
So, N.S., M.A. Ostrowski, and S.D. Gray-Owen, Vigorous response of human innate functioning IgM memory B cells upon infection by Neisseria gonorrhoeae. J Immunol, 2012. 188(8): p. 4008–22.
Heath, E., et al., Epstein-Barr virus infection of naive B cells in vitro frequently selects clones with mutated immunoglobulin genotypes: implications for virus biology. PLoS Pathog, 2012. 8(5): p. e1002697.
Cantaert, T., et al., Increased numbers of CD5+ B lymphocytes with a regulatory phenotype in spondylarthritis. Arthritis Rheum, 2012. 64(6): p. 1859–68.
Toapanta, F.R., P.J. Bernal, and M.B. Sztein, Diverse phosphorylation patterns of B cell receptor-associated signaling in naive and memory human B cells revealed by phosphoflow, a powerful technique to study signaling at the single cell level. Front Cell Infect Microbiol, 2012. 2: p. 128.
Jansen, M.A., et al., Decreased memory B cells and increased CD8 memory T cells in blood of breastfed children: the generation R study. PLoS One, 2015. 10(5): p. e0126019.
Castaneda, D.M., D.M. Salgado, and C.F. Narvaez, B cells naturally induced during dengue virus infection release soluble CD27, the plasma level of which is associated with severe forms of pediatric dengue. Virology, 2016. 497: p. 136–145.
Wu, X., et al., Impaired T Cell-dependent Humoral Immune Response Associated with Juvenile-onset Recurrent Respiratory Papillomatosis Progression. Sci Rep, 2016. 6: p. 36378.
Nakayama, Y., et al., Aiolos Overexpression in Systemic Lupus Erythematosus B Cell Subtypes and BAFF-Induced Memory B Cell Differentiation Are Reduced by CC-220 Modulation of Cereblon Activity. J Immunol, 2017. 199(7): p. 2388–2407.
Anolik, J.H., et al., Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol, 2008. 180(2): p. 688–92.
Tian, C., et al., Immunodominance of the VH1–46 antibody gene segment in the primary repertoire of human rotavirus-specific B cells is reduced in the memory compartment through somatic mutation of nondominant clones. J Immunol, 2008. 180(5): p. 3279–88.
Ghannam, A., et al., Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol, 2008. 181(7): p. 5158–66.
Palanichamy, A., et al., Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol, 2009. 182(10): p. 5982–93.
Berkowska, M.A., et al., Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood, 2011. 118(8): p. 2150–8.
Morbach, H., et al., Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis. Arthritis Rheum, 2011. 63(11): p. 3458–66.
Wu, Y.C., D. Kipling, and D.K. Dunn-Walters, The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol, 2011. 2: p. 81.
Labuda, L.A., et al., Alterations in peripheral blood B cell subsets and dynamics of B cell responses during human schistosomiasis. PLoS Negl Trop Dis, 2013. 7(3): p. e2094.
Degauque, N., et al., Characterization of antigen-specific B cells using nominal antigen-coated flow-beads. PLoS One, 2013. 8(12): p. e84273.
Zhang, L., et al., Plasmacytoid dendritic cells mediate synergistic effects of HIV and lipopolysaccharide on CD27+ IgD- memory B cell apoptosis. J Virol, 2014. 88(19): p. 11430–41.
Bagnara, D., et al., A Reassessment of IgM Memory Subsets in Humans. J Immunol, 2015. 195(8): p. 3716–24.
Czarnowicki, T., et al., Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol, 2016. 137(1): p. 118–129 e5.
Hayashi, M., et al., IL-10-producing regulatory B cells are decreased in patients with psoriasis. J Dermatol Sci, 2016. 81(2): p. 93–100.
Mensah, F., et al., Extended B cell phenotype in patients with myalgic encephalomyelitis/chronic fatigue syndrome: a cross-sectional study. Clin Exp Immunol, 2016. 184(2): p. 237–47.
Woda, M., et al., Dynamics of Dengue Virus (DENV)-Specific B Cells in the Response to DENV Serotype 1 Infections, Using Flow Cytometry With Labeled Virions. J Infect Dis, 2016. 214(7): p. 1001–9.
Martins, C., et al., Pregnancy alters the circulating B cell compartment in atopic asthmatic women, and transitional B cells are positively associated with the development of allergy manifestations in their progeny. Am J Reprod Immunol, 2016. 76(6): p. 465–474.
Colonna-Romano, G., et al., A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev, 2009. 130(10): p. 681–90.
Jacobi, A.M., et al., Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum, 2010. 62(1): p. 201–10.
Weller, S., et al., IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood, 2012. 120(25): p. 4992–5001.
Clemente, A., et al., Increased STAT3 phosphorylation on CD27(+) B-cells from common variable immunodeficiency disease patients. Clin Immunol, 2015. 161(2): p. 77–88.
Corneth, O.B.J., et al., Enhanced Bruton's Tyrosine Kinase Activity in Peripheral Blood B Lymphocytes From Patients With Autoimmune Disease. Arthritis Rheumatol, 2017. 69(6): p. 1313–1324.
Torigoe, M., et al., Metabolic Reprogramming Commits Differentiation of Human CD27(+)IgD(+) B Cells to Plasmablasts or CD27(-)IgD(-) Cells. J Immunol, 2017. 199(2): p. 425–434.
Hu, F., et al., Impaired CD27(+)IgD(+) B Cells With Altered Gene Signature in Rheumatoid Arthritis. Front Immunol, 2018. 9: p. 626.
Morbach, H., et al., Reference values for B cell subpopulations from infancy to adulthood. Clin Exp Immunol, 2010. 162(2): p. 271–9.
Lin, W., et al., Circulating plasmablasts/plasma cells: a potential biomarker for IgG4-related disease. Arthritis Res Ther, 2017. 19(1): p. 25.
Bennett, M.S., et al., Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol, 2017. 102(5): p. 1261–1269.
Statistics
Statistical analyses were conducted using SPSS software version 19.0 (IBM Corporation, Armonk, NY, USA). The Mann‐Whitney U‐test was used for comparisons between the two study groups. Two-tailed (p-values without an asterisk) or one-tailed (p-values with an asterisk) p-values were reported. The Friedman test was used for comparisons between decidual tissues and blood samples collected from the same patients. For patient demographics, the Kruskall‐Wallis test was performed for continuous variables and the Fisher’s exact test for nominal variables. A p‐value <0.05 was considered statistically significant.
RESULTS
In the absence of acute or chronic chorioamnionitis, total B cells are more abundant in the decidua parietalis of women who delivered preterm than in those who delivered at term, regardless of the process of labor
Figure 1A shows the spatial localization of the decidua basalis and decidua parietalis. The gating strategy used to identify total B cells is shown in Figure 1B. First, we compared the proportion of total B cells in the decidual tissues to those in the maternal blood and cord blood. The frequency of B cells in the decidua basalis and decidua parietalis was lower than that observed in maternal blood and cord blood (Figure 1C). Most of the B cells present in the decidua basalis and decidua parietalis co-expressed CD20, as did the B cells in maternal blood and cord blood (Figure 1D). Additionally, a CD19+CD20- subset was present in decidual tissues but rarely found in maternal blood and cord blood (Figure 1D). The number of B cells in the decidua basalis and decidua parietalis ranged from 2×102 to 2×105 cells (Supplementary Figure 1), considerably lower than previously reported 118. Yet, our finding is consistent with previous studies indicating that the B-cell population is a small fraction among decidual leukocytes 85, 123. Preterm placentas are significantly smaller than term placentas and the amount of decidual tissue available varies among patients; therefore, flow cytometry quantification may not allow calculation of the absolute number of decidual B cells. For this reason, we used frequencies, also referred to as proportions, throughout the study.
In the decidua basalis, the total number of B cells did not vary among the term and preterm groups (TNL vs TIL vs PTNL vs PTL, Figure 1E). Further, no differences were observed among those groups when samples were allocated into subgroups comparing acute and chronic inflammatory lesions of the placenta (Figure 1F). In the decidua parietalis, the total number of B cells did not vary among term and preterm groups when placental inflammation was not considered (TNL vs TIL vs PTNL vs PTL, Figure 1G). In the absence of acute or chronic chorioamnionitis, the decidua parietalis of women who delivered preterm had higher proportions of B cells than those who delivered at term, regardless of the presence of labor (PTNL or PTL vs TNL or TIL, Figure 1H). The data indicate that B cells are more abundant in preterm gestation than at term, consistent with findings previously reported 100. Yet, contrary to what has been reported 77, 118, the processes of preterm and term labor did not alter the frequency of total B cells. In the decidua parietalis, B cells were more abundant in women with chronic chorioamnionitis who underwent labor at term compared to those without this placental lesion (TIL with CCA vs TIL or TIL with ACA, Figure 1H). However, no differences were observed in B-cell frequencies between the PTL and PTL with CCA groups.
Identification of different B-cell subsets in the decidual tissues
Next, we performed an extensive literature review of the markers used to identify the main B-cell subsets (Table 3). Two main B-cell subsets have been described: B1 and B2 B cells 124 (Figure 2A). Both B1 and B2 B cells can become transitional B cells, naïve B cells, class-switched memory B cells, non-class-switched memory B cells, and plasmablasts 124 (Figure 2B). CD19+ B cells were subdivided into the above-mentioned B-cell subsets. The gating strategy used to identify these B-cell subsets is shown in Figure 2C. We found that B2 B cells were more abundant in the decidual tissues than B1 B cells, which made up a distinct but very small population (data not shown). Therefore, the B1 B cells were considered by themselves. A t-SNE plot showing the different B-cell subsets identified in the decidual tissues is shown in Figure 2D. Transitional and naïve B cells were the most abundant subsets in the decidual tissues (Figure 2D).
Figure 2. B-cell subsets in the decidua basalis and decidua parietalis.
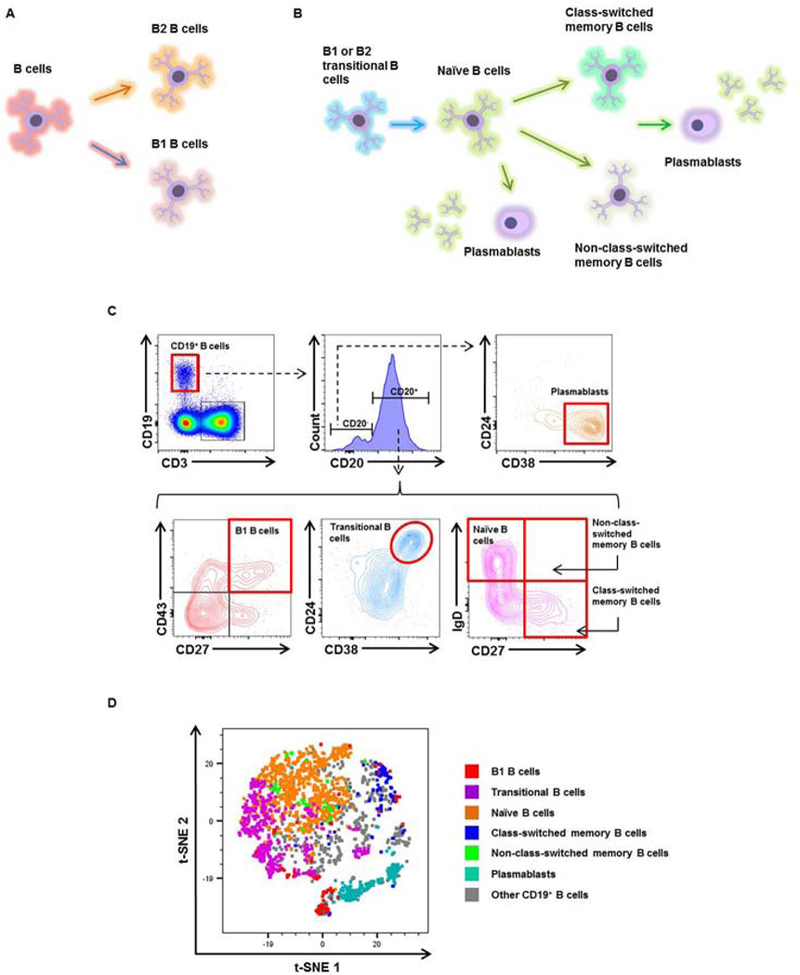
(A)Schematic representation of B1 and B2 B cells. (B) Schematic representation of the B-cell subsets identified in the decidual tissues. (C) Flow cytometry gating strategy used to identify the following B-cell subsets: B1 B cells (CD3-CD19+CD20+CD27+CD43+ cells); transitional B cells (CD3-CD19+CD20+CD38hiCD24hi cells); naïve B cells (CD3-CD19+CD20+CD27-IgD+ cells); class-switched memory B cells (CD3-CD19+CD20+CD27+IgD- cells); non-class-switched memory B cells (CD3-CD19+CD20+CD27+IgD+ cells); and plasmablasts (CD3-CD19+CD20-CD38+CD24- cells). (D) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing B-cell subsets in the decidual tissues. Red = B1 B cells, purple = transitional B cells, orange = naïve B cells, blue = class-switched memory B cells, green = non-class-switched memory B cells, turquoise = plasmablasts, and grey = other CD19+ B cells.
B1 B cells are increased in the decidual tissues of women with chronic chorioamnionitis who underwent labor at term or preterm labor
B1 B cells were rarely found in the decidual tissues, as shown in the t-SNE plot (Figure 3A). In the decidua basalis, the frequency of B1 B cells did not change between the labor and no labor groups (TNL vs TIL and PTNL vs PTL, Figure 3B). A higher proportion of B1 B cells was observed in women who underwent preterm labor compared to those who underwent labor at term (PTL vs TIL, Figure 3B). This difference was most likely driven by the presence of chronic chorioamnionitis in the preterm labor group (PTL with CCA, Figure 3C). The presence of chronic chorioamnionitis also increased the proportion of B1 B cells in labor at term (TIL with CCA vs TIL or TIL with ACA, Figure 3C). In the decidua parietalis, B1 B cells were less abundant in women who underwent labor at term compared to those who delivered at term without labor (TIL vs TNL, Figure 3D). However, women who underwent labor at term with chronic chorioamnionitis had higher proportions of B1 B cells than those without this placental lesion (TIL with CCA vs TIL, Figure 3E). Women who underwent preterm labor with chronic chorioamnionitis also had higher proportions of B1 B cells compared to those without this placental lesion (PTL with CCA vs PTL or PTL with ACA, Figure 3B). These results show that B1 B cells are increased in the decidual tissues of women who underwent labor at term or preterm labor in the presence of chronic chorioamnionitis, but not in those with acute chorioamnionitis or without placental lesions.
Figure 3. B1 B cells in the decidua basalis and decidua parietalis.
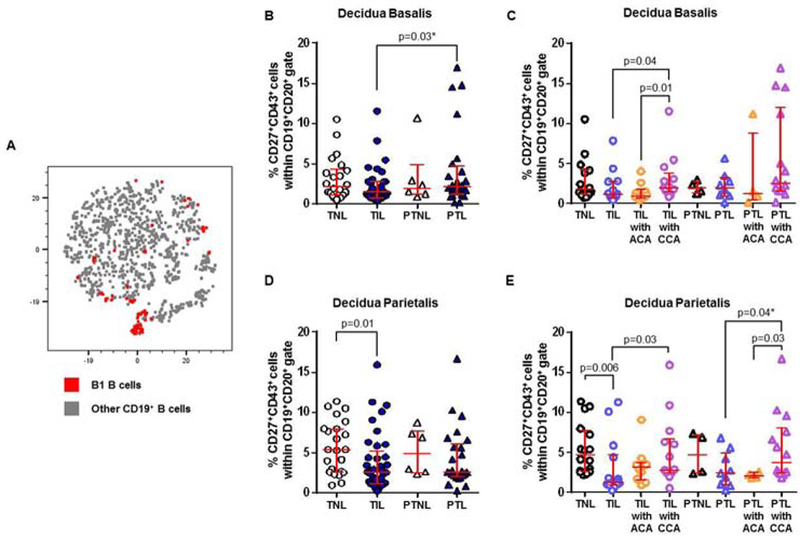
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing B1 B cells in the decidual tissues. Red = B1 B cells and grey = other CD19+ B cells. The proportions of B1 B cells in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of B1 B cells in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Transitional B cells were reduced in the decidual tissues of women with preterm labor compared to gestational age-matched controls
Transitional B cells are the critical link between bone marrow immature and mature B cells present in the peripheral repertoire 125, 126. The transitional B-cell subset is thought to represent a key negative selection checkpoint for autoreactive B cells 127, 128. Transitional B cells were one of the most abundant B-cell subsets present in the decidual tissues, as shown in the t-SNE plot (Figure 4A). In the decidua basalis, the proportion of transitional B cells was lower in women who underwent preterm labor compared to those who delivered preterm in the absence of labor (PTL vs PTNL, Figure 4B). Additionally, transitional B cells were less abundant in women who underwent preterm labor with or without acute or chronic chorioamnionitis compared to those who delivered preterm without labor (PTL with CCA, PTL with ACA, and PTL vs PTNL, Figure 4C). However, transitional B cells were more abundant in women who delivered preterm without labor than in those who delivered at term in the absence of labor (PTNL vs TNL, Figure 4B). In the decidua parietalis, transitional B cells were less abundant in women who underwent preterm labor than in those who delivered preterm without labor (PTL vs PTNL, Figure 4D). Transitional B cells were even fewer in women who underwent preterm labor with chronic chorioamnionitis than in those without this placental lesion (PTL with CCA vs PTL, Figure 4E). Taken together, these data show that transitional B cells are reduced in women with preterm labor compared to gestational age-matched controls.
Figure 4. Transitional B cells in the decidua basalis and decidua parietalis.
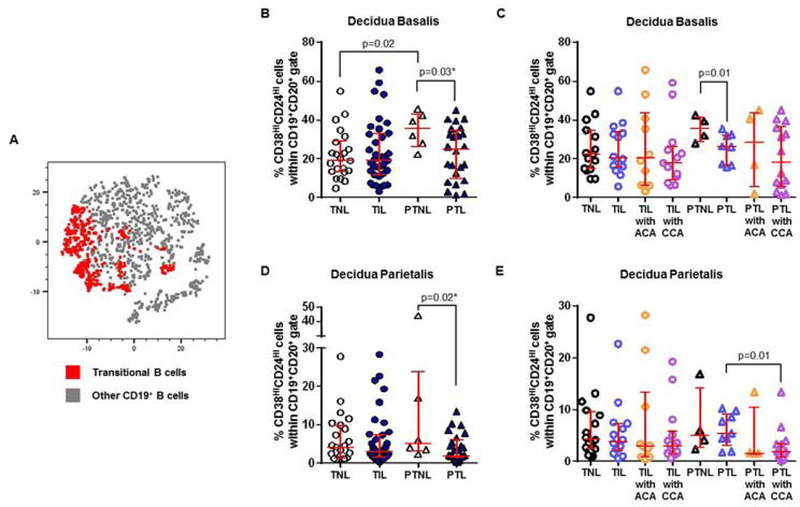
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing transitional B cells in the decidual tissues. Red = transitional B cells and grey = other CD19+ B cells. The proportions of transitional B cells in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of transitional B cells in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Naïve B cells undergo mild alterations in the decidual tissues
Naïve B cells were another abundant B-cell subset found in the decidual tissues, as shown in the t-SNE plot (Figure 5A). In the decidua basalis, naïve B cells did not vary among the study groups (TNL vs TIL vs PTNL vs PTL, Figure 5B). In the absence of placental lesions, naïve B cells were modestly higher in women who delivered preterm without labor than in those who delivered at term without labor (PTNL vs TNL, Figure 5C). In the decidua parietalis, naïve B cells were reduced in women who underwent preterm labor compared to those who delivered preterm without labor (PTL vs PTNL, Figure 5D). This reduction was driven by the presence of chronic chorioamnionitis given that women who underwent preterm labor with this placental lesion tended to have fewer naïve B cells in the decidua parietalis (Figure 5E). Women with preterm labor and chronic chorioamnionitis had fewer naïve B cells than those with labor at term and this placental lesion (PTL with CCA vs TIL with CCA, Figure 5E). In summary, naïve B cells undergo mild alterations in the decidua basalis of women with preterm labor and chronic chorioamnionitis.
Figure 5. Naïve B cells in the decidua basalis and decidua parietalis.
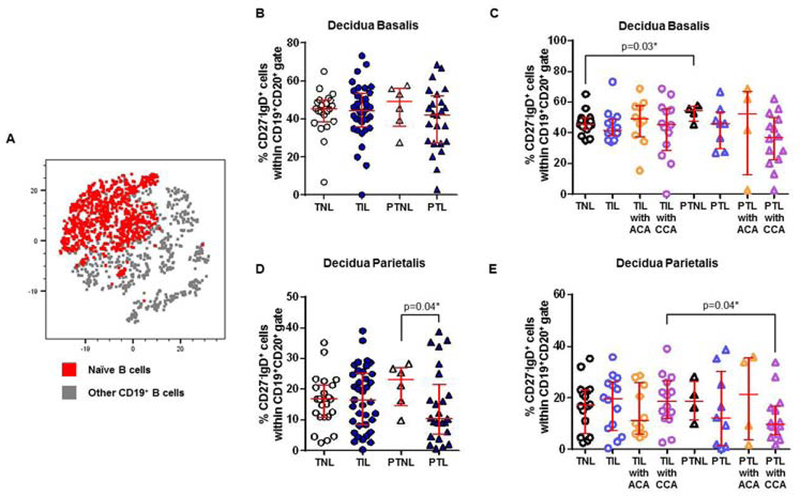
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing naïve B cells in the decidual tissues. Red = naïve B cells and grey = other CD19+ B cells. The proportions of naïve B cells in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of naïve B cells in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Class-switched and non-class-switched memory B cells are rare and undergo mild alterations in the decidual tissues
Naïve B cells can undergo class-switch recombination generating class-switched or non-class-switched memory B cells 129. Next, we examined whether these B-cell subsets were present in the decidual tissues. Class-switched and non-class-switched memory B cells were rarely present in the decidual tissues (Figures 6A and 7A). In the decidua basalis, class-switched memory B cells were more abundant in women who underwent preterm labor than in those with labor at term (PTL vs TIL, Figure 6B). This increase was most likely due to the presence of chronic chorioamnionitis since women who underwent preterm labor with this placental lesion tended to have higher proportions of class-switched memory B cells than those without it (PTL with CCA vs PTNL, PTL, or PTL with ACA, Figure 6C). In the absence of placental lesions, class-switched memory B cells were less abundant in women who underwent labor at term (TIL vs TNL, Figure 6C). However, class-switched memory B cells were more abundant in women who underwent labor at term with chronic chorioamnionitis than in those with acute chorioamnionitis (TIL with CCA vs TIL with ACA, Figure 6C). In the decidua parietalis, class-switched memory B cells were less abundant in women with labor at term than in those who delivered at term without labor (TIL vs TNL, Figure 6D), which was consistently observed in the absence of placental lesions (Figure 6E). No differences were observed between women who delivered preterm with and without labor (PTNL vs PTL, Figure 6D). In summary, in the absence of placental lesions, class-switched memory B cells are rare in the decidual tissues and undergo mild alterations with the presence of labor and/or placental inflammation.
Figure 6. Class-switched memory B cells in the decidua basalis and decidua parietalis.
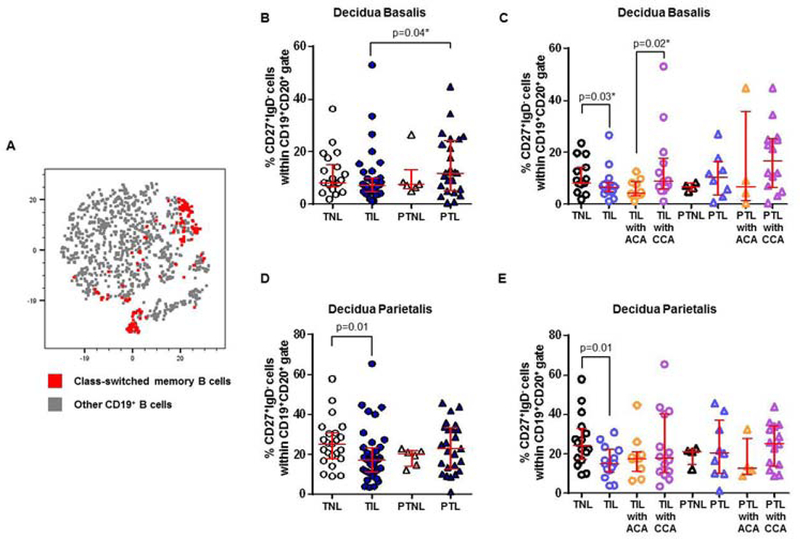
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing class-switched memory B cells in the decidual tissues. Red = class-switched memory B cells and grey = other CD19+ B cells. The proportions of class-switched memory B cells in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of class-switched memory B cells in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Figure 7. Non-class-switched memory B cells in the decidua basalis and decidua parietalis.
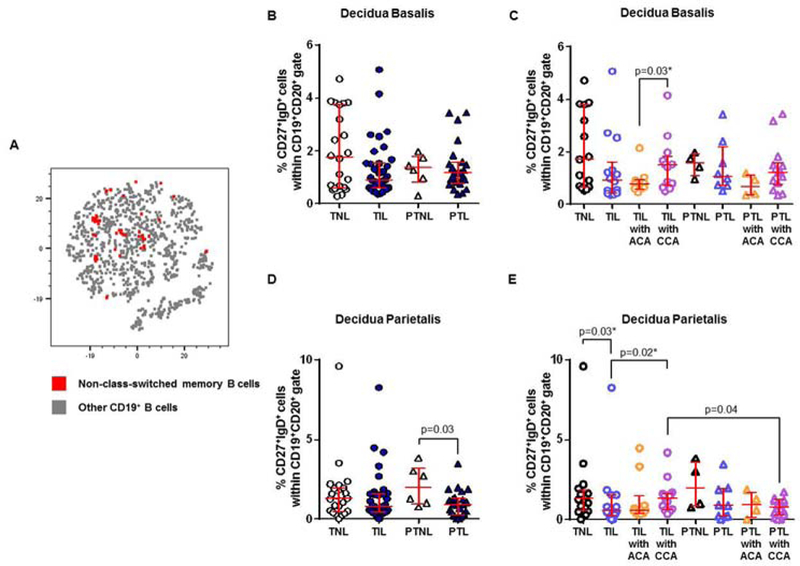
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing non-class-switched memory B cells in the decidual tissues. Red = non-class-switched memory B cells and grey = other CD19+ B cells. The proportions of non-class-switched memory B cells in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of non-class-switched memory B cells in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
Non-class-switched memory B cells were even less abundant than class-switched memory B cells in the decidual tissues (Figure 7A). In the decidua basalis, non-class-switched memory B cells did not vary among the study groups (TNL vs TIL vs PTNL vs PTL, Figure 7B). When the patients were divided into subgroups according to the presence of placental lesions, a slight increase in the proportion of non-class-switched memory B cells was observed in women who underwent labor at term with chronic chorioamnionitis compared to those with acute chorioamnionitis (TIL with CCA vs TIL with ACA, Figure 7C). In the decidua parietalis, non-class-switched memory B cells were less abundant in women who underwent preterm labor than those who delivered preterm without labor (PTL vs PTNL, Figure 7D). This was most likely driven by the presence of chronic chorioamnionitis since women with this placental lesion had lower proportions of this B-cell subset than those without it (PTL with CCA vs PTL and PTNL, Figure 7E). In the absence of placental lesions, a small reduction in the proportion of non-class-switched memory B cells was also observed in women with labor at term compared to those without labor (TIL vs TNL, Figure 7E). However, women who underwent labor at term with chronic chorioamnionitis had greater proportions of non-class-switched memory B cells than those without this placental lesion (TIL with CCA vs TIL, Figure 7E). Therefore, in the absence of placental lesions, non-class-switched memory B cells are rare in the decidual tissues and undergo mild alterations with the presence of labor and/or placental inflammation.
Plasmablasts seemed to increase in the decidual tissues of women with chronic chorioamnionitis who underwent labor at term or preterm labor
Naïve B cells and memory B cells can differentiate into plasmablasts 129, 130. We then investigated the presence of plasmablasts in the decidual tissues. Plasmablasts were a relatively abundant population in the decidual tissues (Figure 8A). In the decidua basalis and decidua parietalis, plasmablasts did not vary among the study groups (TNL vs TIL vs PTNL vs PTL, Figures 8B & 8D). When the samples were divided into subgroups according to the presence of placental lesions, we consistently found that women who underwent labor at term with chronic chorioamnionitis had higher proportions of plasmablasts than those without this placental lesion in both the decidua basalis and decidua parietalis (TIL with CCA vs TIL or TIL with ACA, Figures 8C & 8E). Plasmasblasts seemed to be more abundant in women who underwent preterm labor with CCA than in those without this placental lesion (Figures 8C & 8E). Taken together, these data show that plasmablasts are abundant in the decidual tissues of women who underwent labor at term or preterm labor with chronic chorioamnionitis.
Figure 8. Plasmablasts in the decidua basalis and decidua parietalis.
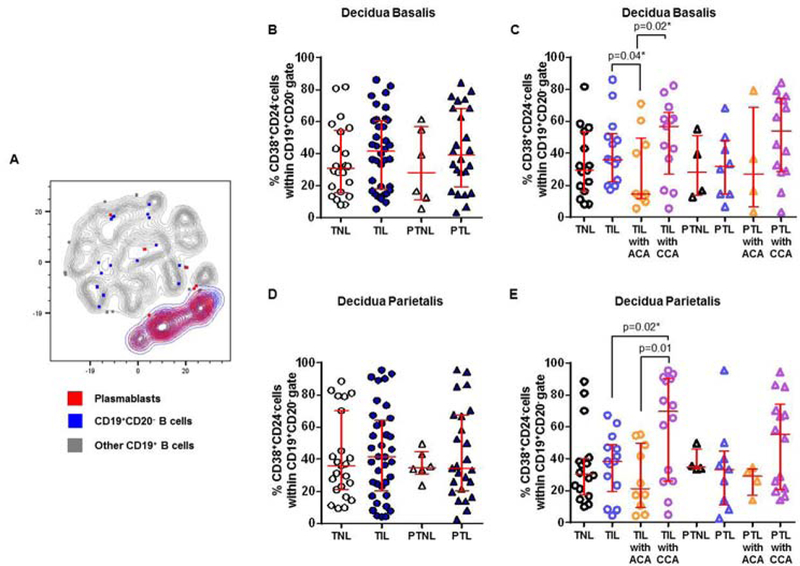
(A) A representative t-distributed stochastic neighbor embedding (t-SNE) dot plot visualizing plasmablasts in the decidual tissues. Red = plasmablasts, blue = CD19+CD20- B cells, and grey = other CD19+ B cells. The proportions of plasmablasts in the decidua basalis (B) or decidua parietalis (D) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. The TIL and PTL patients were subdivided into those with acute histologic chorioamnionitis (ACA) or chronic histologic chorioamnionitis (CCA), and those without these lesions. Non-labor controls without ACA or CCA were included as well. The proportions of plasmablasts in the decidua basalis (C) or decidua parietalis (E) in these patient subgroups. N = 4 – 16 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
B cells in the decidual tissues expressed high levels of IL-12, IL-6 and/or IL35
Recent studies indicated that, besides production of antibodies, B cells participate in immune responses by producing inflammatory cytokines 131, 132. We investigated whether decidual B cells produce pro- and anti-inflammatory cytokines that are associated with the process of labor. In the decidua basalis and decidua parietalis, a small proportion of B cells expressed cytokines (Figure 9A & 9C). In the decidua basalis, B cells expressed higher levels of interleukin (IL)-12 and IL-35 than IFNγ, IL-2, IL-4, IL-6, IL-10, and TNFα (Figure 9B). In the decidua parietalis, B cells expressed higher levels of IL-12 and IL-6 than other cytokines (Figure 9D). These results show that B cells participate in the inflammatory milieu at the maternal-fetal interface.
Figure 9. Cytokine expression by decidual B cells. (A & C).
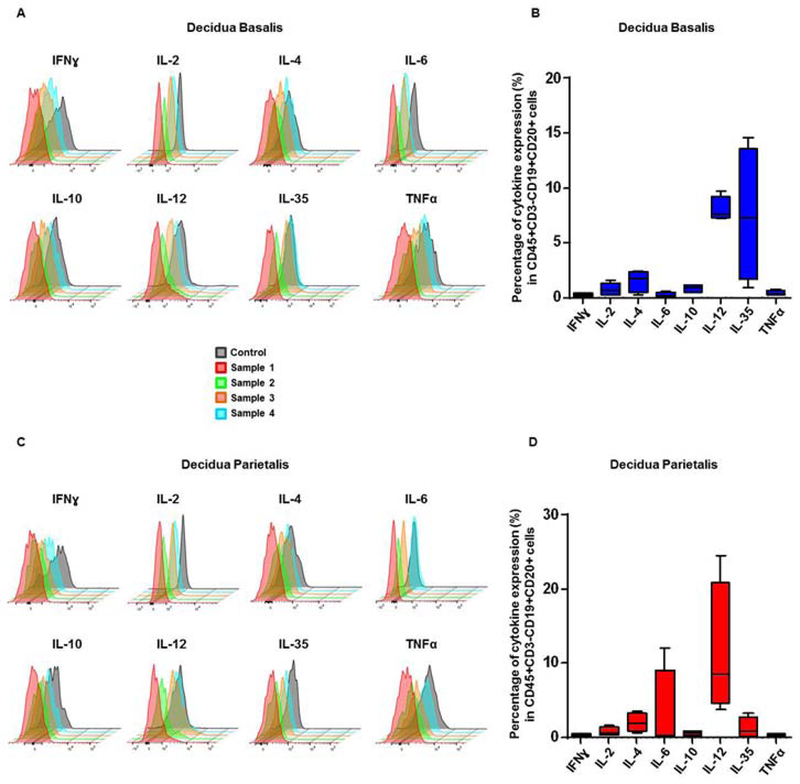
Staggered offset overlay histograms of cytokines expressed by B cells (CD45+CD3-CD19+CD20+ cells) in the decidua basalis and parietalis. Four representative samples of decidual B cells isolated from women who delivered preterm or term. Control histograms represent signals derived from isotypes or autofluorescence controls. (B & D) Proportions of B cells expressing IFNγ, IL-2, IL-4, IL-6, IL-10, IL-12, IL-35, and TNFα in the decidua basalis and parietalis.
DISCUSSION
Principal findings
The principal findings of this study are as follows: 1) In the absence of acute or chronic chorioamnionitis, total B cells were more abundant in the decidua parietalis of women who delivered preterm than in those who delivered at term, regardless of the presence of labor; 2) decidual transitional and naïve B cells were the most abundant B-cell subsets; 3) decidual B1 B cells were increased in women with labor at term or preterm labor with chronic chorioamnionitis compared to those without this placental lesion; 4) decidual transitional B cells were reduced in women with preterm labor compared to those without labor; 5) decidual naïve B cells underwent mild alterations with the process of preterm labor and/or placental inflammation; 6) class-switched and non-class-switched memory B cells were rare and underwent mild alterations with the process of labor and/or placental inflammation; 7) decidual plasmablasts seemed to increase in women with labor at term or preterm with chronic chorioamnionitis; and 8) decidual B cells expressed high levels of IL-12, IL-6, or IL-35. These findings indicate that specific B-cell populations at the maternal-fetal interface undergo alterations in a subset of women with labor at term and preterm labor and chronic chorioamnionitis.
In the absence of acute or chronic chorioamnionitis, total B cells were more abundant in the decidua parietalis of women who delivered preterm than in those who delivered at term, regardless of the presence of labor
Herein, we found that total B cells were not increased in the decidua basalis and decidua parietalis of women who underwent labor at term compared to those who delivered at term without labor. This finding is consistent, in part, with what has previously been published. We and other investigators have reported that there are no differences in the proportion of B cells in the decidua parietalis of women who delivered at term with and without labor 77, 85. However, previous studies have shown that the decidua basalis of women who underwent spontaneous labor at term had greater proportions of total B cells than those who delivered at term without labor 77. The discrepancy between the above-mentioned study and our findings could be explained by the fact that we considered only viable B cells. These findings show that the frequency of total viable B cells is not altered with the physiological process of labor at term.
We also found that total B cells were not increased in the decidua basalis and decidua parietalis of women who underwent preterm labor compared to those who delivered preterm in the absence of labor. To our knowledge, it is the first time that this comparison between preterm labor cases and gestational age-matched controls has been made. A previous study reported that the abundance of B cells in the decidua parietalis was significantly increased in women who underwent spontaneous preterm labor compared to those who underwent spontaneous labor at term 118. However, this comparison warrants caution given that gestational age may influence the proportion of B cells found in the decidual tissues 100. Without considering acute and chronic chorioamnionitis, no differences in the frequency of total B cells in the decidua parietalis were observed between women who underwent preterm labor and those with term labor. Women with preterm labor, but without acute or chronic chorioamnionitis, had higher frequencies of decidual B cells compared to those with labor at term. Nonetheless, this increase in the proportion of total B cells was also observed by comparing women who delivered preterm in the absence of labor to those who delivered at term without labor. Therefore, these data indicate that the process of preterm labor does not increase the proportion of total B cells in the decidua parietalis; rather, it is an effect of gestational age.
After the two study groups were subdivided based on the presence of acute or chronic chorioamnionitis, we found that the frequency of total B cells in the decidua basalis did not significantly vary. Yet, differences in B-cell proportions in the decidua parietalis were observed among the study groups. This result is explained by the fact that acute and chronic chorioamnionitis are diagnosed in the chorioamniotic membranes, located next to the decidua parietalis 94, 121. Of interest, the presence of chronic chorioamnionitis increased the frequency of total B cells in the decidua parietalis of women with labor at term but not in those with preterm labor (Figure 1H). These findings suggest that the process of chronic chorioamnionitis in labor at term is distinct from that observed in women with preterm labor.
Different B-cell subsets were present in the decidual tissues; yet, we focused our discussion on the main differences observed in women with labor at term and those with preterm labor.
Decidual B1 B cells are increased in women with chronic chorioamnionitis who underwent labor at term or preterm labor
B1 B cells show a skewed antigen receptor repertoire toward self-antigens, as well as tonic B-cell receptor intracellular signaling, spontaneous secretion of IgM, and efficient T-cell stimulation 133. Herein, we found that B1 B cells are increased in the decidua basalis and decidua parietalis of women with labor at term or preterm labor with chronic chorioamnionitis. These data suggest, for the first time, a role for decidual B1 B cells in the pathological process of chronic chorioamnionitis associated with either labor at term or preterm labor. Potentially, B1 B cells could be participating in the stimulation of CD8+ cytotoxic T cells, which can induce apoptosis of trophoblasts leading to maternal anti-fetal rejection 94. More recently, we found that both decidual CD4+ and CD8+ T cells from women who underwent spontaneous preterm labor displayed an effector memory phenotype and expressed high levels of granzyme B and perforin 97, molecules capable of cytotoxicity 134–140. B1 B cells constitutively secrete IL-10 141, suggesting that these cells are implicated in the regulation of such a chronic inflammatory process associated with labor at term and preterm labor. Given that B1 B cells are either pro-inflammatory (through cytotoxic CD8+ T-cell stimulation) or anti-inflammatory (through IL-10 secretion), their role may vary in chronic chorioamnionitis and requires further investigation.
Decidual naïve B cells undergo mild alterations with the process of preterm labor and/or placental inflammation
During a primary response, transitional B cells differentiate to naïve B cells that upon a secondary response can generate memory B cells 142. Naïve B cells and memory B cells display differential expression of IgD or CD27 and in vitro function following BCR stimulation 143–145. Overall, naïve B cells present antigen-inexperienced responses compared to memory B cells 145. Naïve B cells can also proliferate into short-lived plasmablasts or plasma cells that produce low-affinity antibodies 146. In the current study, we found that naïve B cells were reduced in patients with preterm labor and chronic chorioamnionitis, but no differences were observed in memory B cells. Yet, decidual tissues from women who underwent preterm labor with chronic chorioamnionitis tended to have greater proportions of plasmablasts. These data suggest that, in women with preterm labor and chronic chorioamnionitis, naïve B cells might proliferate to plasmablasts in the decidual tissues. However, additional investigation of decidual B cells is required to strengthen this proposed concept.
Decidual plasmablasts increase in women who underwent labor at term or preterm labor with chronic chorioamnionitis
Plasmablasts are newly differentiated B cells that can leave the lymphoid organs and home in either tissue or bone marrow 147, 148. Additionally, they are capable of differentiating into fully mature plasma cells 149. Plasmablasts are usually short-lived and can be generated inside and outside of the germinal centers 150. In general, plasmablasts represent an accessible source of mature antibodies characterized by the regulation of several transcription factors, such as BLIMP1 (B-lymphocyte-induced maturation protein 1) and IRF4 (interferon-regulatory factor 4) 151. Our results showed that plasmablasts seemed to increase in women who underwent labor at term or preterm labor with chronic chorioamnionitis. These data suggest that, in addition to T cells, the hallmark of chronic chorioamnionitis 94, B-cell antibody-mediated responses are implicated in the chronic inflammatory process associated with preterm labor and birth.
B cells can express cytokines in the decidual tissues
Cytokine production by B cells is implicated in multiple aspects of immunity 132. Thus, B cells can express pro- and anti-inflammatory cytokines to mediate immune responses 131, 132. Herein, we found that, in both the decidua basalis and decidua parietalis, B cells expressed high levels of IL-12, a cytokine that increased in the amniotic fluid of women with preterm labor and preterm birth 46. In the decidua parietalis, B cells also expressed high levels of IL-6, a cytokine released by gestational tissues and highly relevant in the pathological process of preterm labor 26, 152. Therefore, these data provide evidence that, by releasing cytokines, B cells at the maternal-fetal interface contribute to the pro-inflammatory microenvironment associated with labor. B cells, however, could also be participating in the regulation of such a hostile microenvironment, given that these cells also expressed a high level of IL-35, an immunosuppressive cytokine previously reported to be expressed by the placental tissues 153. Further studies are needed to investigate whether decidual B cells display different cytokine profiles than those derived from gestational-aged matched controls.
Conclusion
The current study provides the characterization of B-cell subsets at the human maternal-fetal interface during labor at term and preterm labor. In the absence of acute or chronic chorioamnionitis, total B cells are more abundant in the decidua parietalis of women who delivered preterm than in those who delivered at term, regardless of the process of labor. Yet, an increase in the proportions of B1 B cells and plasmablasts was observed in women who underwent labor at term or preterm labor with chronic chorioamnionitis compared to those without this placental lesion. Decidual B cells are capable of producing pro- and anti-inflammatory cytokines. In conclusion, the B-cell compartment at the maternal-fetal interface undergoes alterations in women with labor at term or preterm labor and chronic chorioamnionitis, suggesting that these adaptive immune cells are implicated in the process of labor associated with chronic inflammation of the placenta. Additional experimentation is required to investigate the functionality of chronic chorioamnionitis-derived B cells.
Supplementary Material
The number of CD19+ B cells in the decidua basalis (A) and decidua parietalis (B) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.
ACKNOWLEDGEMENTS:
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit, as well as the research assistants from the PRB Clinical Laboratory, for their help in collecting samples. We also thank staff members of the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R: Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muglia LJ, Katz M: The enigma of spontaneous preterm birth. N Engl J Med 2010;362:529–535. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM: The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–429. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Dey SK, Fisher SJ: Preterm labor: one syndrome, many causes. Science 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC: Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–279. [PubMed] [Google Scholar]
- 6.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ: An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–1667. [DOI] [PubMed] [Google Scholar]
- 7.Gomez R, Romero R, Edwin SS, David C: Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am 1997;11:135–176. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Gotsch F, Pineles B, Kusanovic JP: Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65:S194–202. [DOI] [PubMed] [Google Scholar]
- 9.Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L: Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldenow E, Gendrin C, Ngo L, Bierle C, Vornhagen J, Coleman M, Merillat S, Armistead B, Whidbey C, Alishetti V, Santana-Ufret V, Ogle J, Gough M, Srinouanprachanh S, MacDonald JW, Bammler TK, Bansal A, Liggitt HD, Rajagopal L, Adams Waldorf KM: Group B Streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss JF 3rd, Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, Pearson LN, York TP, Schenkein HA: Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018;218:294–314 e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM, Gomez-Lopez N: Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol 2018;9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Presicce P, Park CW, Senthamaraikannan P, Bhattacharyya S, Jackson C, Kong F, Rueda CM, DeFranco E, Miller LA, Hildeman DA, Salomonis N, Chougnet CA, Jobe AH, Kallapur SG: IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keelan JA: Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol 2018;125:89–99. [DOI] [PubMed] [Google Scholar]
- 15.Bobitt JR, Ledger WJ: Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med 1977;19:8–12. [PubMed] [Google Scholar]
- 16.Bobitt JR, Ledger WJ: Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet Gynecol 1978;51:56–62. [PubMed] [Google Scholar]
- 17.Miller JM Jr., Pupkin MJ, Hill GB: Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol 1980;136:796–804. [DOI] [PubMed] [Google Scholar]
- 18.Wallace RL, Herrick CN: Amniocentesis in the evaluation of premature labor. Obstet Gynecol 1981;57:483–486. [PubMed] [Google Scholar]
- 19.Bobitt JR, Hayslip CC, Damato JD: Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol 1981;140:947–952. [DOI] [PubMed] [Google Scholar]
- 20.Wahbeh CJ, Hill GB, Eden RD, Gall SA: Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol 1984;148:739–743. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Mazor M: Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–584. [DOI] [PubMed] [Google Scholar]
- 22.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC: Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–824. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Avila C, Brekus CA, Morotti R: The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci 1991;622:355–375. [DOI] [PubMed] [Google Scholar]
- 24.Watts DH, Krohn MA, Hillier SL, Eschenbach DA: The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–357. [DOI] [PubMed] [Google Scholar]
- 25.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, Shim SS: The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol 2001;185:1137–1142. [DOI] [PubMed] [Google Scholar]
- 26.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK: Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Espinoza J, Chaiworapongsa T, Kalache K: Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–274. [DOI] [PubMed] [Google Scholar]
- 28.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH: Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–1345. [DOI] [PubMed] [Google Scholar]
- 29.Lee SE, Romero R, Park CW, Jun JK, Yoon BH: The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633 e631–638. [DOI] [PubMed] [Google Scholar]
- 30.Lee SE, Romero R, Lee SM, Yoon BH: Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med 2010;38:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, Yoon BH: The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? J Matern Fetal Neonatal Med 2012;25:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L: A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014;71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacora P, Romero R, Erez O, Maymon E, Panaitescu B, Kusanovic JP, Tarca AL, Hsu CD, Hassan SS: The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med 2017:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM: Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L: Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM: Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, Gomez-Lopez N, Romero R, Yoon BH: Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017;216:604 e601–604 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD: Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol 2018;80:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R, Mazor M, Tartakovsky B: Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–971. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Tartakovsky B: The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–1045. [DOI] [PubMed] [Google Scholar]
- 41.Friel LA, Romero R, Edwin S, Nien JK, Gomez R, Chaiworapongsa T, Kusanovic JP, Tolosa JE, Hassan SS, Espinoza J: The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med 2007;35:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, Erez O, Edwin S, Schmidt AM: Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med 2008;36:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, Romero R: Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med 2008;21:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS: Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS: Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med 2012;25:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, Margolis L: Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015;213:836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS: Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, Gomez-Lopez N: HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Lopez N, Romero R, Plazyo O, Schwenkel G, Garcia-Flores V, Unkel R, Xu Y, Leng Y, Hassan SS, Panaitescu B, Cha J, Dey SK: Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol 2017;217:592 e591–592 e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, Mor G: Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–1355. [DOI] [PubMed] [Google Scholar]
- 51.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, Espinoza J, Erez O, Nhan-Chang CL, Than NG, Vaisbuch E, Hassan SS: CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 2008;60:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS: Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G: Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 2010;185:1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ilievski V, Hirsch E: Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod 2010;83:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrahams VM: The role of the Nod-like receptor family in trophoblast innate immune responses. J Reprod Immunol 2011;88:112–117. [DOI] [PubMed] [Google Scholar]
- 56.Lappas M: NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod 2013;89:14. [DOI] [PubMed] [Google Scholar]
- 57.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, Beaman KD: Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 2013;191:5702–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koga K, Izumi G, Mor G, Fujii T, Osuga Y: Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol 2014;72:192–205. [DOI] [PubMed] [Google Scholar]
- 59.Agrawal V, Jaiswal MK, Ilievski V, Beaman KD, Jilling T, Hirsch E: Platelet-activating factor: a role in preterm delivery and an essential interaction with Toll-like receptor signaling in mice. Biol Reprod 2014;91:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, Drewlo S, Tarca AL, Hassan SS, Gomez-Lopez N: Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol 2016;196:1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, Gomez-Lopez N: An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol 2016;196:2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N: An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol 2016;13:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Lopez N, Romero R, Leng Y, Garcia-Flores V, Xu Y, Miller D, Hassan SS: Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Negishi Y, Shima Y, Takeshita T, Takahashi H: Distribution of invariant natural killer T cells and dendritic cells in late pre-term birth without acute chorioamnionitis. Am J Reprod Immunol 2017;77. [DOI] [PubMed] [Google Scholar]
- 65.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, Hassan SS: Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci 2017;24:1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS: A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci 2017;24:1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, Panaitescu B: Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol 2017;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musilova I, Andrys C, Krejsek J, Drahosova M, Zednikova B, Pliskova L, Zemlickova H, Jacobsson B, Kacerovsky M: Amniotic fluid pentraxins: Potential early markers for identifying intra-amniotic inflammatory complications in preterm pre-labor rupture of membranes. Am J Reprod Immunol 2017. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, Maymon E: Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693 e691–693 e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS, Gomez-Lopez N: Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol 2018;79:e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Varrey A, Romero R, Panaitescu B, Miller D, Chaiworapongsa T, Patwardhan M, Faro J, Pacora P, Hassan SS, Hsu CD, Gomez-Lopez N: Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol 2018;80:e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paquette AG, Shynlova O, Kibschull M, Price ND, Lye SJ, Global Alliance to Prevent P, Stillbirth Systems Biology of Preterm Birth T: Comparative analysis of gene expression in maternal peripheral blood and monocytes during spontaneous preterm labor. Am J Obstet Gynecol 2018;218:345 e341–345 e330. [DOI] [PubMed] [Google Scholar]
- 73.Miller D, Motomura K, Garcia-Flores V, Romero R, Gomez-Lopez N: Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front Immunol 2018;9:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vargas ML, Santos JL, Ruiz C, Montes MJ, Aleman P, Garcia-Tortosa C, Garcia-Olivares E: Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol 1993;29:135–140. [DOI] [PubMed] [Google Scholar]
- 75.Bonney EA, Pudney J, Anderson DJ, Hill JA: Gamma-delta T cells in midgestation human placental villi. Gynecol Obstet Invest 2000;50:153–157. [DOI] [PubMed] [Google Scholar]
- 76.Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, Claas F: Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol 2003;49:261–268. [DOI] [PubMed] [Google Scholar]
- 77.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH: Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 2004;62:125–137. [DOI] [PubMed] [Google Scholar]
- 78.Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, Scherjon SA: Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta 2006;27 Suppl A:S47–53. [DOI] [PubMed] [Google Scholar]
- 79.Constantin CM, Masopust D, Gourley T, Grayson J, Strickland OL, Ahmed R, Bonney EA: Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol 2007;179:4383–4389. [DOI] [PubMed] [Google Scholar]
- 80.Tilburgs T, Scherjon SA, Roelen DL, Claas FH: Decidual CD8+CD28- T cells express CD103 but not perforin. Hum Immunol 2009;70:96–100. [DOI] [PubMed] [Google Scholar]
- 81.Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SA, Claas FH: Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol 2009;80:22–32. [DOI] [PubMed] [Google Scholar]
- 82.Norton MT, Fortner KA, Oppenheimer KH, Bonney EA: Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology 2010;131:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tilburgs T, Schonkeren D, Eikmans M, Nagtzaam NM, Datema G, Swings GM, Prins F, van Lith JM, van der Mast BJ, Roelen DL, Scherjon SA, Claas FH: Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol 2010;185:4470–4477. [DOI] [PubMed] [Google Scholar]
- 84.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, Vadillo-Ortega F: Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 2011;205:235 e215–224. [DOI] [PubMed] [Google Scholar]
- 85.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F: Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013;69:212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shepard MT, Bonney EA: PD-1 regulates T cell proliferation in a tissue and subset-specific manner during normal mouse pregnancy. Immunol Invest 2013;42:385–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonney EA: Alternative theories: Pregnancy and immune tolerance. J Reprod Immunol 2017;123:65–71. [DOI] [PubMed] [Google Scholar]
- 88.Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, Kilby MD: Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J Immunol 2017;199:3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Zwan A, Bi K, Norwitz ER, Crespo AC, Claas FHJ, Strominger JL, Tilburgs T: Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A 2018;115:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS: The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010;23:1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ: A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One 2011;6:e16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS: Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol 2012;67:184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J, Kim JS, Park JW, Park CW, Park JS, Jun JK, Yoon BH: Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta 2013;34:681–689. [DOI] [PubMed] [Google Scholar]
- 94.Kim CJ, Romero R, Chaemsaithong P, Kim JS: Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213:S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Ahn H, Panaitescu B, Vadillo-Ortega F, Sanchez-Torres C, Salisbury KS, Hassan SS: In vivo T-cell activation by a monoclonal alphaCD3epsilon antibody induces preterm labor and birth. Am J Reprod Immunol 2016;76:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frascoli M, Coniglio L, Witt R, Jeanty C, Fleck-Derderian S, Myers DE, Lee TH, Keating S, Busch MP, Norris PJ, Tang Q, Cruz G, Barcellos LF, Gomez-Lopez N, Romero R, MacKenzie TC: Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Ahn H, Done B, Hassan S, Hsu CD, Sanchez-Torres C, Gomez-Lopez N: Effector and activated T cells Induce Preterm Labor and Birth that is Prevented by Treatment with Progesterone. J Immunol 2018:Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulmer JN, Sunderland CA: Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology 1984;52:349–357. [PMC free article] [PubMed] [Google Scholar]
- 99.Haller H, Radillo O, Rukavina D, Tedesco F, Candussi G, Petrovic O, Randic L: An immunohistochemical study of leucocytes in human endometrium, first and third trimester basal decidua. J Reprod Immunol 1993;23:41–49. [DOI] [PubMed] [Google Scholar]
- 100.Bartmann C, Segerer SE, Rieger L, Kapp M, Sutterlin M, Kammerer U: Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol 2014;71:109–119. [DOI] [PubMed] [Google Scholar]
- 101.Redline RW, Lu CY: Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest 1989;61:27–36. [PubMed] [Google Scholar]
- 102.Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC: Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol Reprod 2013;89:90. [DOI] [PubMed] [Google Scholar]
- 103.Muzzio D, Zenclussen AC, Jensen F: The role of B cells in pregnancy: the good and the bad. Am J Reprod Immunol 2013;69:408–412. [DOI] [PubMed] [Google Scholar]
- 104.Rolle L, Memarzadeh Tehran M, Morell-Garcia A, Raeva Y, Schumacher A, Hartig R, Costa SD, Jensen F, Zenclussen AC: Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol 2013;70:448–453. [DOI] [PubMed] [Google Scholar]
- 105.Zenclussen AC: Adaptive immune responses during pregnancy. Am J Reprod Immunol 2013;69:291–303. [DOI] [PubMed] [Google Scholar]
- 106.Fettke F, Schumacher A, Costa SD, Zenclussen AC: B cells: the old new players in reproductive immunology. Front Immunol 2014;5:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muzzio D, Zygmunt M, Jensen F: The role of pregnancy-associated hormones in the development and function of regulatory B cells. Front Endocrinol (Lausanne) 2014;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muzzio DO, Soldati R, Ehrhardt J, Utpatel K, Evert M, Zenclussen AC, Zygmunt M, Jensen F: B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol Reprod 2014;91:115. [DOI] [PubMed] [Google Scholar]
- 109.Muzzio DO, Soldati R, Rolle L, Zygmunt M, Zenclussen AC, Jensen F: B-1a B cells regulate T cell differentiation associated with pregnancy disturbances. Front Immunol 2014;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bommer I, Muzzio DO, Zygmunt M, Jensen F: Progesterone and estradiol exert an inhibitory effect on the production of anti-inflammatory cytokine IL-10 by activated MZ B cells. J Reprod Immunol 2016;116:113–116. [DOI] [PubMed] [Google Scholar]
- 111.Fettke F, Schumacher A, Canellada A, Toledo N, Bekeredjian-Ding I, Bondt A, Wuhrer M, Costa SD, Zenclussen AC: Maternal and Fetal Mechanisms of B Cell Regulation during Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While Alpha-Fetoprotein Drives Them into Apoptosis. Front Immunol 2016;7:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muzzio DO, Ziegler KB, Ehrhardt J, Zygmunt M, Jensen F: Marginal zone B cells emerge as a critical component of pregnancy well-being. Reproduction 2016;151:29–37. [DOI] [PubMed] [Google Scholar]
- 113.Wolfson ML, Muzzio DO, Ehrhardt J, Franchi AM, Zygmunt M, Jensen F: Expression analysis of cannabinoid receptors 1 and 2 in B cells during pregnancy and their role on cytokine production. J Reprod Immunol 2016;116:23–27. [DOI] [PubMed] [Google Scholar]
- 114.Schumacher A, Ehrentraut S, Scharm M, Wang H, Hartig R, Morse HC 3rd, Zenclussen AC: Plasma Cell Alloantigen 1 and IL-10 Secretion Define Two Distinct Peritoneal B1a B Cell Subsets With Opposite Functions, PC1(high) Cells Being Protective and PC1(low) Cells Harmful for the Growing Fetus. Front Immunol 2018;9:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ziegler KB, Muzzio DO, Matzner F, Bommer I, Ventimiglia MS, Malinowsky K, Ehrhardt J, Zygmunt M, Jensen F: Human pregnancy is accompanied by modifications in B cell development and immunoglobulin profile. J Reprod Immunol 2018;129:40–47. [DOI] [PubMed] [Google Scholar]
- 116.Rinaldi SF, Makieva S, Saunders PT, Rossi AG, Norman JE: Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol Hum Reprod 2017;23:708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Solders M, Gorchs L, Gidlof S, Tiblad E, Lundell AC, Kaipe H: Maternal Adaptive Immune Cells in Decidua Parietalis Display a More Activated and Coinhibitory Phenotype Compared to Decidua Basalis. Stem Cells Int 2017;2017:8010961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huang B, Faucette AN, Pawlitz MD, Pei B, Goyert JW, Zhou JZ, El-Hage NG, Deng J, Lin J, Yao F, Dewar RS 3rd, Jassal JS, Sandberg ML, Dai J, Cols M, Shen C, Polin LA, Nichols RA, Jones TB, Bluth MH, Puder KS, Gonik B, Nayak NR, Puscheck E, Wei WZ, Cerutti A, Colonna M, Chen K: Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med 2017;23:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Society for Pediatric Pathology PSAFINC: Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–448. [DOI] [PubMed] [Google Scholar]
- 120.Redline RW: Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 2006;11:296–301. [DOI] [PubMed] [Google Scholar]
- 121.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM: Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Redline RW: Classification of placental lesions. Am J Obstet Gynecol 2015;213:S21–28. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y, Plazyo O, Romero R, Hassan SS, Gomez-Lopez N: Isolation of Leukocytes from the Human Maternal-fetal Interface. J Vis Exp 2015:e52863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Montecino-Rodriguez E, Dorshkind K: B-1 B cell development in the fetus and adult. Immunity 2012;36:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung JB, Silverman M, Monroe JG: Transitional B cells: step by step towards immune competence. Trends Immunol 2003;24:343–349. [DOI] [PubMed] [Google Scholar]
- 126.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH: Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 2009;182:5982–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carsetti R, Kohler G, Lamers MC: Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med 1995;181:2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R: B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med 1999;190:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weisel F, Shlomchik M: Memory B Cells of Mice and Humans. Annu Rev Immunol 2017;35:255–284. [DOI] [PubMed] [Google Scholar]
- 130.Seifert M, Kuppers R: Human memory B cells. Leukemia 2016;30:2283–2292. [DOI] [PubMed] [Google Scholar]
- 131.Lund FE: Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol 2008;20:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shen P, Fillatreau S: Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol 2015;15:441–451. [DOI] [PubMed] [Google Scholar]
- 133.Griffin DO, Holodick NE, Rothstein TL: Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med 2011;208:67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ: How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol 1998;10:581–587. [DOI] [PubMed] [Google Scholar]
- 135.Trapani JA, Smyth MJ: Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2002;2:735–747. [DOI] [PubMed] [Google Scholar]
- 136.Trambas CM, Griffiths GM: Delivering the kiss of death. Nat Immunol 2003;4:399–403. [DOI] [PubMed] [Google Scholar]
- 137.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ: Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004;104:2840–2848. [DOI] [PubMed] [Google Scholar]
- 138.Rock MT, Yoder SM, Wright PF, Talbot TR, Edwards KM, Crowe JE Jr.: Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol 2005;174:3757–3764. [DOI] [PubMed] [Google Scholar]
- 139.Takata H, Takiguchi M: Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol 2006;177:4330–4340. [DOI] [PubMed] [Google Scholar]
- 140.Lin L, Couturier J, Yu X, Medina MA, Kozinetz CA, Lewis DE: Granzyme B secretion by human memory CD4 T cells is less strictly regulated compared to memory CD8 T cells. BMC Immunol 2014;15:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alhakeem SS, Sindhava VJ, McKenna MK, Gachuki BW, Byrd JC, Muthusamy N, Bondada S: Role of B cell receptor signaling in IL-10 production by normal and malignant B-1 cells. Ann N Y Acad Sci 2015;1362:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moens L, Kane A, Tangye SG: Naive and memory B cells exhibit distinct biochemical responses following BCR engagement. Immunol Cell Biol 2016;94:774–786. [DOI] [PubMed] [Google Scholar]
- 143.Jelinek DF, Splawski JB, Lipsky PE: Human peripheral blood B lymphocyte subpopulations: functional and phenotypic analysis of surface IgD positive and negative subsets. J Immunol 1986;136:83–92. [PubMed] [Google Scholar]
- 144.Liu YJ, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J: Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7–1 and B7–2. Immunity 1995;2:239–248. [DOI] [PubMed] [Google Scholar]
- 145.Tangye SG, Tarlinton DM: Memory B cells: effectors of long-lived immune responses. Eur J Immunol 2009;39:2065–2075. [DOI] [PubMed] [Google Scholar]
- 146.Claes N, Fraussen J, Stinissen P, Hupperts R, Somers V: B Cells Are Multifunctional Players in Multiple Sclerosis Pathogenesis: Insights from Therapeutic Interventions. Front Immunol 2015;6:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, Popova L, Kaminiski D, Fucile CF, Albizua I, Kyu S, Chiang KY, Bradley KT, Burack R, Slifka M, Hammarlund E, Wu H, Zhao L, Walsh EE, Falsey AR, Randall TD, Cheung WC, Sanz I, Lee FE: Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015;43:132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Landsverk OJ, Snir O, Casado RB, Richter L, Mold JE, Reu P, Horneland R, Paulsen V, Yaqub S, Aandahl EM, Oyen OM, Thorarensen HS, Salehpour M, Possnert G, Frisen J, Sollid LM, Baekkevold ES, Jahnsen FL: Antibody-secreting plasma cells persist for decades in human intestine. J Exp Med 2017;214:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leandro MJ: B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther 2013;15 Suppl 1:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.De Silva NS, Klein U: Dynamics of B cells in germinal centres. Nat Rev Immunol 2015;15:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klein U, Dalla-Favera R: Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 2008;8:22–33. [DOI] [PubMed] [Google Scholar]
- 152.Prins JR, Gomez-Lopez N, Robertson SA: Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol 2012;95:1–14. [DOI] [PubMed] [Google Scholar]
- 153.Mao H, Gao W, Ma C, Sun J, Liu J, Shao Q, Song B, Qu X: Human placental trophoblasts express the immunosuppressive cytokine IL-35. Hum Immunol 2013;74:872–877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of CD19+ B cells in the decidua basalis (A) and decidua parietalis (B) from women who delivered at term with labor (TIL) or without labor (TNL) and women who delivered preterm with labor (PTL) or without labor (PTNL). N = 6 – 37 per group. Red midlines and whiskers indicate medians and interquartile ranges, respectively.


