Abstract
Problem:
Acute atherosis is a uteroplacental arterial lesion that is associated with pregnancy complications such as preeclampsia and preterm birth, the leading cause of perinatal morbidity and mortality worldwide. However, the immunobiology of acute atherosis is poorly understood.
Method of study:
Placental basal plate samples were collected from women who delivered with (n = 11) and without (n = 31) decidua basalis lesions of acute atherosis. Multi-color flow cytometry was used to quantify M1- and M2-like macrophage subsets and the expression of iNOS and IL-12 by decidual macrophages. Multiplex fluorescence staining and phenoptics were performed to localize M1-, MOX-, and Mhem-like macrophages in the decidual basalis.
Results:
Macrophages displayed diverse phenotypes in the decidua basalis with acute atherosis. M2-like macrophages were the most abundant subset in the decidua; yet, this macrophage subset did not change with the presence of acute atherosis. Decidual M1-like macrophages were increased in acute atherosis, and such macrophages displayed a pro-inflammatory phenotype, as indicated by the expression of iNOS and IL-12. Decidual M1-like pro-inflammatory macrophages were localized near both transformed and non-transformed vessels in the decidua basalis with acute atherosis. MOX and Mhem macrophages were also identified near transformed vessels in the decidua basalis with acute atherosis. Finally, monocyte-like cells were present on the vessel wall of non-transformed decidual vessels, indicating a possible intravascular source for macrophages in acute atherosis.
Conclusions:
These findings provide a molecular foundation for future mechanistic inquiries about the role of pro-inflammatory macrophages in the pathogenesis of acute atherosis.
Keywords: inflammation, innate immunity, monocytes, phenoptics, preeclampsia, preterm labor, spiral artery, pregnancy
GRAPHICAL ABSTRACT
INTRODUCTION
During normal pregnancy, the spiral arteries in the placental bed undergo a physiologic transformation into dilated, thinly-walled, less tortuous vessels capable of providing increased blood flow to the placental intervillous space1–3. However, in a subset of pregnant women the spiral arteries fail to undergo complete transformation4, 5, and such vessels are susceptible to the development of uteroplacental lesions such as acute atherosis6–12.
Acute atherosis, named due to its histologic similarity to the atheromatous changes observed in atherosclerosis13, was first described in patients with preeclampsia14 and is associated with multiple pregnancy complications4, 12, 15–25 including preterm labor23. Acute atherosis has been described in the spiral arteries of the decidua underlying the placenta, termed the decidua basalis, and in the decidua parietalis (underlying the fetal chorioamniotic membranes)2, 23, as well as in myometrial tissues26, 27. Such lesions are characterized by the presence of 1) fibrinoid necrosis of the vessel wall, 2) perivascular lymphocytic infiltration, and 3) accumulation of foam cells6–11, 13, 14, 21, 28, 29. The foam cells detected in the decidual tissues from women with acute atherosis have been assumed to be mostly lipid-laden macrophages30; however, other non-foamy macrophages are detected in and around these vessels. The phenotype and function of these macrophages have not been described.
Decidual macrophages contribute to local immune tolerance and play a central role throughout pregnancy31–41. The classical paradigm described a linear range of macrophage polarization towards either a pro-inflammatory phenotype with high phagocytic/anti-tumor capability (M1 macrophages)42–48 or a homeostatic phenotype involved in tissue repair and resolution of inflammation (M2 macrophages)45–47, 49–57. However, the majority of decidual macrophages do not fit the M1-M2 paradigm35, 58. Rather, most of the decidual macrophages display a unique, M2-like phenotype throughout pregnancy31, 32, 34, 36–40, 58–64. Decidual macrophages from women who underwent spontaneous labor at term or preterm labor, however, exhibit an M1-like pro-inflammatory phenotype58. These findings suggest that the decidual microenvironment alters the phenotype of infiltrating macrophages. Of particular interest, acute atherosis is likely associated with decidual inflammation65, 66. Therefore, we hypothesized that macrophages from the decidua basalis with acute atherosis display a pro-inflammatory phenotype that is different from those tissues without this arterial lesion.
The aim of this study was to determine the immunophenotype of macrophages in the decidua basalis with acute atherosis.
MATERIALS AND METHODS
Human subjects, clinical specimens, and definitions
Human placental basal plate samples (maternal side of the placenta, decidua basalis) were obtained at the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA). The collection and utilization of biological materials for research purposes were approved by the Institutional Review Boards of Wayne State University and NICHD. All participating women provided written informed consent. The study groups included women with (n = 11) and without (n = 31) decidua basalis lesions of acute atherosis. Decidua basalis samples from subsets of these women were used separately in different experiments. The demographic and clinical characteristics of the study population are shown in Table 1. Among women with acute atherosis, 45.5% (5/11) had preeclampsia compared to 16.1% (5/31) of women without this lesion (Table 1), which is consistent with a previous report24.
Table 1.
Demographic and clinical characteristics of the study population
| No acute atherosis (n=31) | Acute atherosis(n=11) | p-value | |
|---|---|---|---|
| Acute atherosis of decidua basalis spiral arteriesb | 0 (0/31) | 100 (11/11) | <0.001 |
| Maternal age (years)a | 28 (24.5–31.5) | 30 (23.5–35.5) | 0.7 |
| Pre-pregnancy body mass index (kg/m2)a | 25 (22–29)c |
28.1 (25.3–34.2) |
0.2 |
| Raceb | 0.1 | ||
| African-American | 54.8 (17/31) | 90.9 (10/11) | |
| Caucasian | 22.6 (7/31) | 0 (0/11) | |
| Hispanic | 9.7 (3/31) | 0 (0/11) | |
| Other | 12.9 (4/31) | 9.1 (1/11) | |
| Delivery routeb | 1 | ||
| Vaginal | 41.9 (13/31) | 45.5 (5/11) | |
| Cesarean section | 58.1 (18/31) | 54.5 (6/11) | |
| Preeclampsiab | 16.1 (5/31) | 45.5 (5/11) | 0.09 |
| Gestational age at delivery (weeks)a | 38.4 (36.5–39.1) | 33.7 (31.7–36.2) | 0.001 |
| Birth weighta | 3030 (2385–3445) | 1675 (1427.5–2530) | 0.001 |
Data are given as median (interquartile range) and percentage (n/N)
Kruskal-Wallis test
Fisher’s exact test
Three missing data
Placental histopathological examination
Placentas were examined histologically by a perinatal pathologist blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols23, 24, 67. Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Acute atherosis was diagnosed in the decidua basalis by the presence of fibrinoid necrosis of the spiral artery wall with lipid-laden macrophages in the vessel wall and a perivascular lymphocytic infiltrate23, 24, 67.
Isolation of decidual leukocytes
Decidual leukocytes were isolated from the decidua basalis, as previously described68. Briefly, the decidual tissue was homogenized using a gentleMACS Dissociator (Miltenyi Biotec, San Diego, CA, USA) in StemPro Accutase Cell Dissociation Reagent (Life Technologies, Grand Island, NY, USA). Homogenized tissues were incubated for 45 min at 37°C with gentle agitation. After incubation, tissues were washed in sterile 1X phosphate-buffered saline (PBS) (Life Technologies) and filtered through a 100μm cell strainer (Falcon, Corning Life Sciences, Inc., Durham, NC, USA). The resulting cell suspension was centrifuged at 300 x g for 10 min at 4°C. Decidual leukocytes were then separated by density gradient using the reagent Ficoll-Paque Plus (GE Healthcare Biosciences, Uppsala, Sweden), following the manufacturer’s instructions. Cells collected from the mononuclear layer of the density gradient were washed with 1X PBS and immediately used for immunophenotyping.
Immunophenotyping of decidual macrophages
Isolated decidual mononuclear cells were incubated with 20μl of human FcR blocking reagent (Miltenyi Biotec) in 80μl of stain buffer (Cat#554656, BD Biosciences, San Jose, CA, USA) for 10 min at 4°C. The cells were then incubated with fluorochrome-conjugated anti-human monoclonal antibodies (Supplementary Table 1) for 30 min at 4°C in the dark. After extracellular staining, the cells were washed with 1X PBS to remove excess antibody, resuspended in 0.5 mL stain buffer, and acquired using the BD LSR II Flow Cytometer (BD Biosciences) and BD FACSDiva 6.0 software (BD Biosciences). For intracellular immunophenotyping, following extracellular staining, the cells were fixed and permeabilized using the BD Cytofix/Cytoperm Fixation and Permeabilization Solution (BD Biosciences). Next, the cells were washed with 1X BD Perm/Wash Buffer (BD Biosciences), re-suspended in 50μL of the same buffer, and stained with intracellular antibodies (Supplementary Table 1) for 30 min at 4°C in the dark. Finally, the stained cells were washed with 1X BD Perm/Wash Buffer, re-suspended in 0.5 mL stain buffer, and acquired using the BD LSR II Flow Cytometer and BD FACSDiva 6.0 software. The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA). The analysis and figures were performed using the FlowJo software version 10 (FlowJo, LLC, Ashland, OR, USA). ICAM-3 is downregulated in decidual macrophages compared with blood monocytes34, 36; therefore, we characterized macrophages within the ICAM-3- gate.
Oil-red O staining
Decidua basalis tissues from the maternal site of the placenta were embedded in Tissue-Tek optimum cutting temperature (OCT) compound (Miles, Elkhart, IN, USA) and snap-frozen in liquid nitrogen. Eight-μm-thick sections of OCT-embedded basal plate were cut, fixed with 10% formalin, and rinsed in distilled water. Following incubation in 100% propylene glycol (American MasterTech Scientific Inc., Lodi, CA, USA) for 2 min, tissue sections were stained with oil-red O staining solution (American MasterTech Scientific Inc.) for 45 min at 37°C. After staining, tissue sections were incubated with 85% propylene glycol (Electron Microscopy Sciences, Hatfield, PA, USA) for 5 min and rinsed with distilled water until the water became clear. Stained tissue sections were then counterstained with modified Mayer’s hematoxylin solution (American MasterTech, Lodi, CA, USA), rinsed in distilled water, and mounted with AquaSlip aqueous mounting medium (Cat#MMC0619; American MasterTech). Images were visualized using an Olympus BX60 fluorescence microscope (Olympus, Tokyo, Japan). Pictures were taken using an Olympus DP71 camera and Olympus cellSens Entry software (Olympus).
Multiplex immunofluorescence and phenoptics (i.e. multispectral imaging)
Five-μm-thick sections of formalin-fixed, paraffin-embedded decidua basalis tissues from the maternal site of the placenta were cut and mounted on SuperFrost Plus microscope slides. Multiplex immunofluorescence staining was performed using the Opal 7 kit (Cat#NEL811001KT; PerkinElmer, Waltham, MA, USA), following the manufacturer’s instructions. Prior to multiplex immunofluorescence staining, each analyte was individually optimized with single antibody staining combined with different fluorescent TSA reagents (PerkinElmer). After deparaffinization, slides were placed in antigen retrieval (AR) buffer and boiled using a microwave oven. Following blocking to eliminate non-specific binding, slides were incubated with antibodies against human smooth muscle actin (SMA) (CAT#M0851; Dako North America, Carpinteria, CA, USA), cytokeratin-7 (CK7) (CAT# M7018; Dako North America), CD68 (CAT# M0814; Dako North America), B7–1/CD80 (Cat#MAB140; R&D Systems, Inc., Minneapolis, MN, USA), inducible nitric oxide synthase (iNOS) (CAT#PA3–030A; Life Technologies), interleukin (IL)-12A (CAT#HPA001886, Sigma, St. Louis, MO, USA), heme oxygenase-1 (HMOX-1) (CAT#MA1–112, Invitrogen by Thermo Fisher Scientific, Carlsbad, CA, USA), and CD163 (CAT#bs-2527R, Bioss Antibodies, Inc., Woburn, MA, USA) at room temperature. The slides were then washed and incubated with Opal Polymer HRP Ms+Rb (Cat#ARH1001EA; PerkinElmer). Next, the slides were incubated with one of the following fluorescent TSA reagents (PerkinElmer) included in the Opal 7 kit: Opal 520, Opal 540, Opal 570, Opal 620, Opal 650, or Opal 690 (dilution 1:100). After washing, the slides were counterstained with Spectral DAPI (Cat#FP1490; PerkinElmer) and mounted using ProLong Diamond Anti-fade Mountant (Life Technologies). Unstained tissue sections (autofluorescence controls) and tissue sections stained with isotype (negative controls) were also included. Multiplex staining was performed by consecutively staining slide-mounted tissues using the same antibody concentrations and conditions validated through single-plex staining. Each previous primary and secondary antibody was removed by boiling in AR buffer before the application of the next primary antibody. After multiplex staining, phenoptics was performed in the slides using the Vectra Polaris Multi-spectral Imaging System (PerkinElmer), and images were analyzed and converted to the immunohistochemistry view using the InForm 2.4.1 image analysis software (PerkinElmer).
Statistical analysis
Data were analyzed using IBM SPSS v19.0 (IBM Corporation; Armonk, NY, USA). For patient demographics, the Fisher’s exact test was used to compare proportions among groups and the Kruskal-Wallis test was used for comparing continuous variables among groups. Experimental data was compared between study groups using the Mann-Whitney U-test. t-SNE plots were generated using the FlowJo v10 software. A p-value of ≤0.05 was considered statistically significant.
RESULTS
The immunophenotype of decidual macrophages in acute atherosis
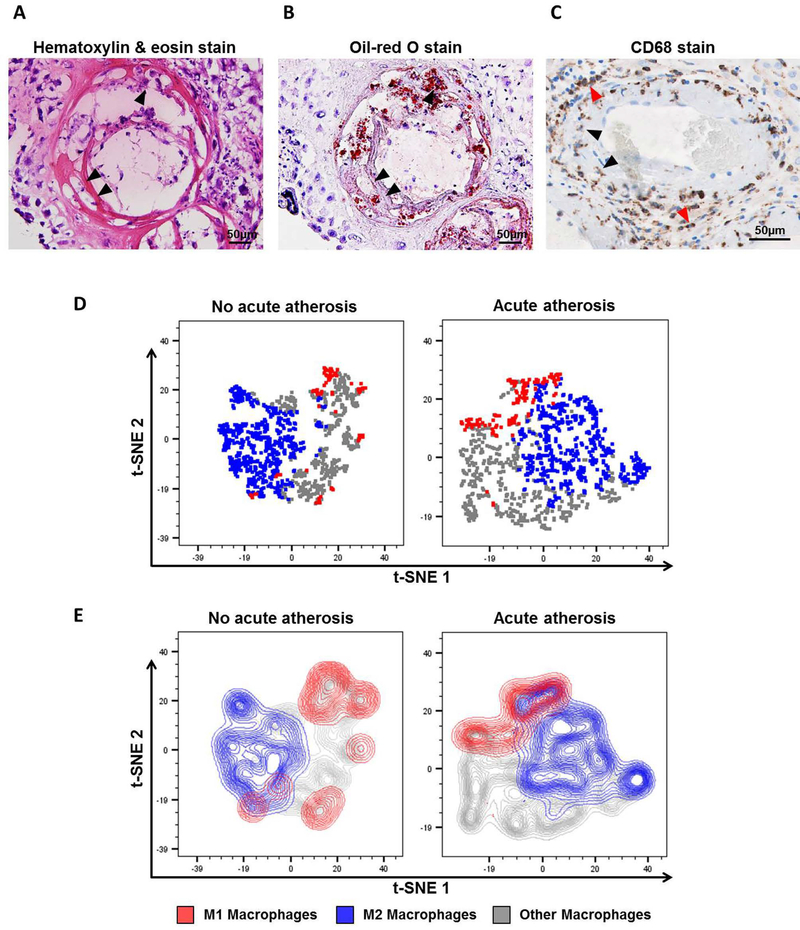
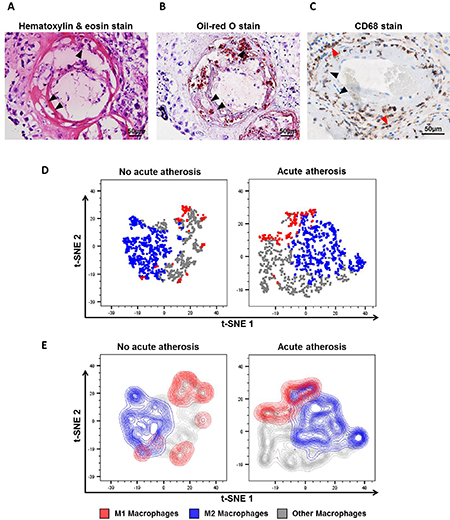
First, we visualized the accumulation of lipid-laden foam cells in the decidual vessels with acute atherosis using hematoxylin & eosin and oil-red O stains (Figures 1A&B, black arrows). These foam cells were positive for CD68, a marker for macrophage scavenger receptors69 that is also expressed by histiocytes70, 71 (Figure 1C, black arrows). Yet, other CD68+ decidual macrophages without lipid accumulation were also localized perivascularly, surrounding the atherotic vessels (Figure 1C, red arrows).
Figure 1. The immunophenotype of decidual macrophages in acute atherosis.
Representative images of histological staining of decidual vessels using (A) hematoxylin & eosin or (B) oil-red O. (C) Representative image of immunohistochemical staining of CD68 to detect macrophages and foam cells. Black arrows indicate foamy macrophages, and red arrows show non-foamy macrophages. Magnification = 200X. Scale bars = 50μm. Flow cytometry analysis of decidual macrophage subsets was performed using t-distributed stochastic neighbor embedding (t-SNE) shown as (D) dot plots or (E) contour plots. Red = M1-like macrophages (CD45+CD14+ICAM-3-CD80+ cells), blue = M2-like macrophages (CD45+CD14+ICAM-3-CD209+CD163+ cells), and grey = non-M1/M2 macrophages.
Next, the immunophenotype of decidual macrophages was assessed using multi-color flow cytometry. Figures 1D&E show t-distributed stochastic neighbor embedding (t-SNE) plots of the macrophage phenotypes identified in the decidua basalis with or without acute atherosis. Three unique populations were detected: M1-like (CD45+CD14+ICAM-3-CD80+ cells), M2-like (CD45+CD14+ICAM-3-CD163+CD209+ cells), and others (neither M1- nor M2-like) (Figures 1D&E). The t-SNE plots clearly showed that M2-like macrophages are more abundant than M1-like macrophages (Figures 1D&E). These results illustrate the diversity of macrophage phenotypes in the decidua basalis.
Acute atherosis is characterized by an increase in M1-like decidual macrophages
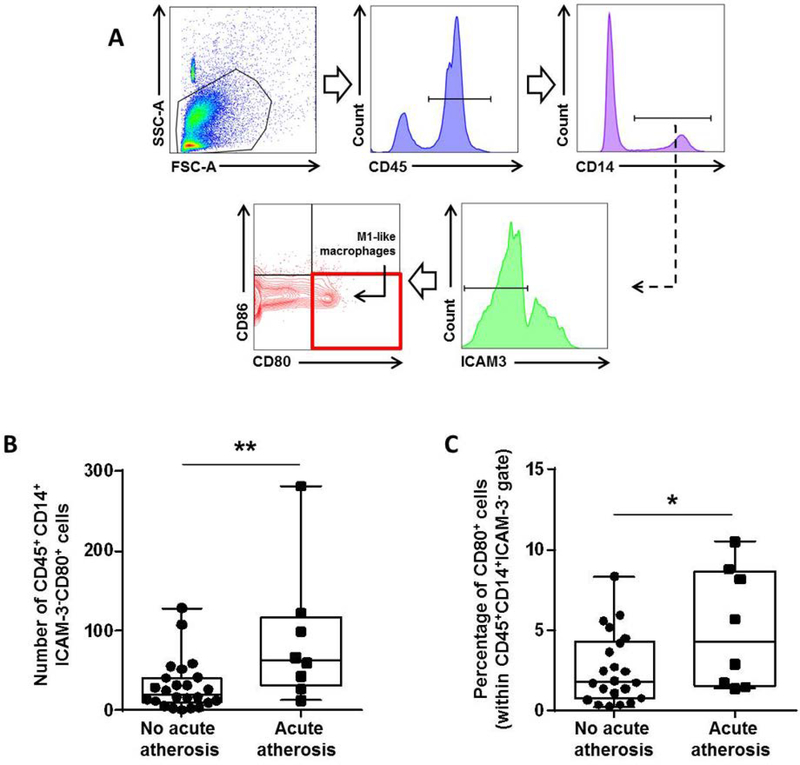
Next, we investigated whether M1-like macrophages were more abundant in the decidua basalis with acute atherosis. The gating strategy used to identify M1-like macrophages in the decidua basalis is shown in Figure 2A. Both the number and proportion of M1-like macrophages were higher in the decidua basalis with acute atherosis compared to those without this lesion (Figures 2B & C). These results suggest that decidual macrophages undergo an M1-like polarization in the pathologic pro-inflammatory microenvironment of acute atherosis.
Figure 2. Acute atherosis is characterized by an increase in M1-like decidual macrophages.
(A) Flow cytometry gating strategy used to identify decidual M1-like macrophages (CD45+CD14+ICAM-3-CD80+ cells). Dot plots illustrate the (B) number and (C) proportion of M1-like macrophages in the decidua basalis with or without acute atherosis. Mid-lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min-max range. Cases with (n=8) or without (n=24) acute atherosis. *p<0.05, **p≤0.01
Acute atherosis is characterized by an increase in pro-inflammatory decidual macrophages
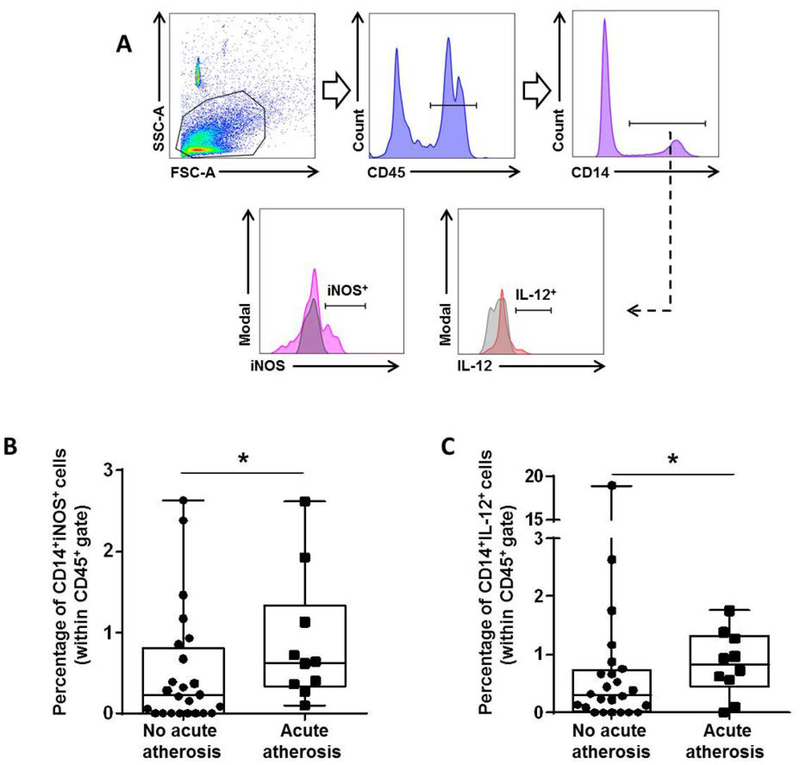
M1 macrophages release pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS) and interleukin (IL)-1272–74. To explore the pro-inflammatory phenotype of decidual macrophages in acute atherosis, we examined the expression of these mediators by flow cytometry (Figure 3A). Consistent with an M1-like phenotype, more macrophages from the decidua basalis with acute atherosis expressed iNOS and IL-12 compared to those without this lesion (Figures 3B&C). These results confirm that macrophages acquire a pro-inflammatory M1-like phenotype in the decidua basalis with acute atherosis.
Figure 3. Acute atherosis is characterized by an increase in pro-inflammatory decidual macrophages.
(A) Flow cytometry gating strategy used to identify decidual macrophages expressing the pro-inflammatory mediators inducible nitric oxide synthase (iNOS) or interleukin (IL)-12 (CD45+CD14+iNOS+ or CD45+CD14+IL-12+ cells). Dot plots illustrate the percentage of (B) CD45+CD14+iNOS+ macrophages and (C) CD45+CD14+IL-12+ macrophages in the decidua basalis with or without acute atherosis. Mid-lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min-max range. Cases with (n=10) or without (n=24) acute atherosis. *p<0.05
Acute atherosis does not alter the M2-like decidual macrophage population
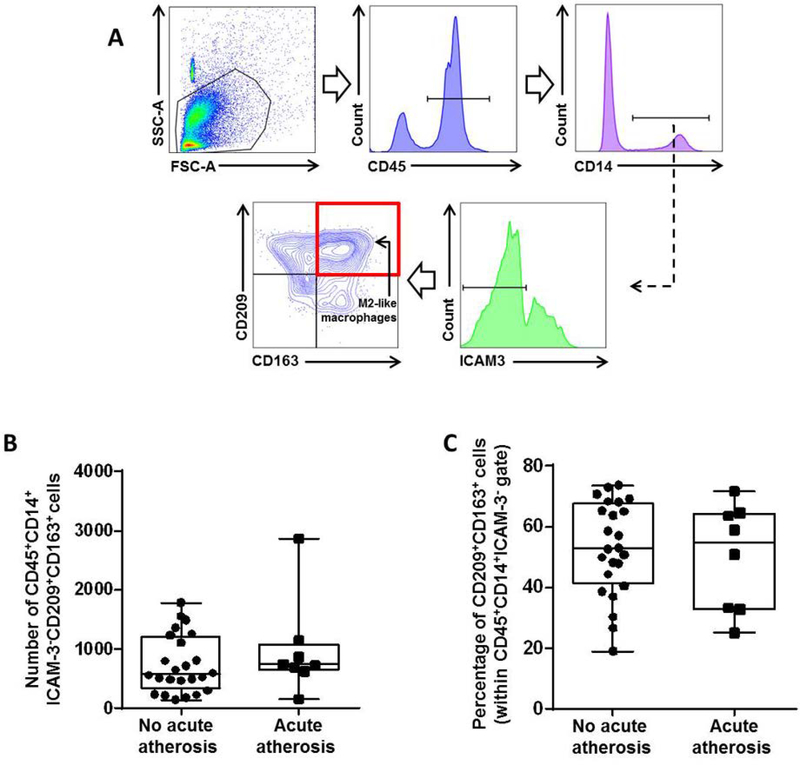
An M1 macrophage polarization can be accompanied by a decrease in M2-polarized macrophages36, 58. Therefore, we next investigated whether the M1-like polarization in acute atherosis was associated with a reduction in M2-like macrophages. The gating strategy used to identify M2-like macrophages in the decidua basalis is shown in Figure 4A. M2-like macrophages were the most abundant phenotype in the decidua basalis, confirming previous studies58. Yet, neither the number nor the proportion of M2-like macrophages was altered in the decidua basalis with acute atherosis (Figures 4B&C). These data indicate that, although M2-like macrophages are abundant, their phenotype does not change during the process of acute atherosis.
Figure 4. Acute atherosis does not alter the M2-like decidual macrophage population.
(A) Flow cytometry gating strategy used to identify decidual M2-like macrophages (CD45+CD14+ICAM-3-CD209+CD163+ cells). Dot plots illustrate the (B) number and (C) proportion of M2-like macrophages in the decidua basalis with or without acute atherosis. Mid-lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min-max range. Cases with (n=8) or without (n=24) acute atherosis.
Immunolocalization of M1-like macrophages in the decidual vessels with acute atherosis
To localize the M1-like macrophages associated with acute atherosis, we performed phenoptics, a novel technology used to immunophenotype cells in tissue sections75–79. Phenoptics allowed us to simultaneously immunolocalize six different markers in nucleated cells (i.e. DAPI-positive cells). The lesions of acute atherosis occur in spiral arteries which fail to fully transform as part of pregnancy adaptations6–11; therefore, we first searched for such non-transformed vessels in the decidua basalis.
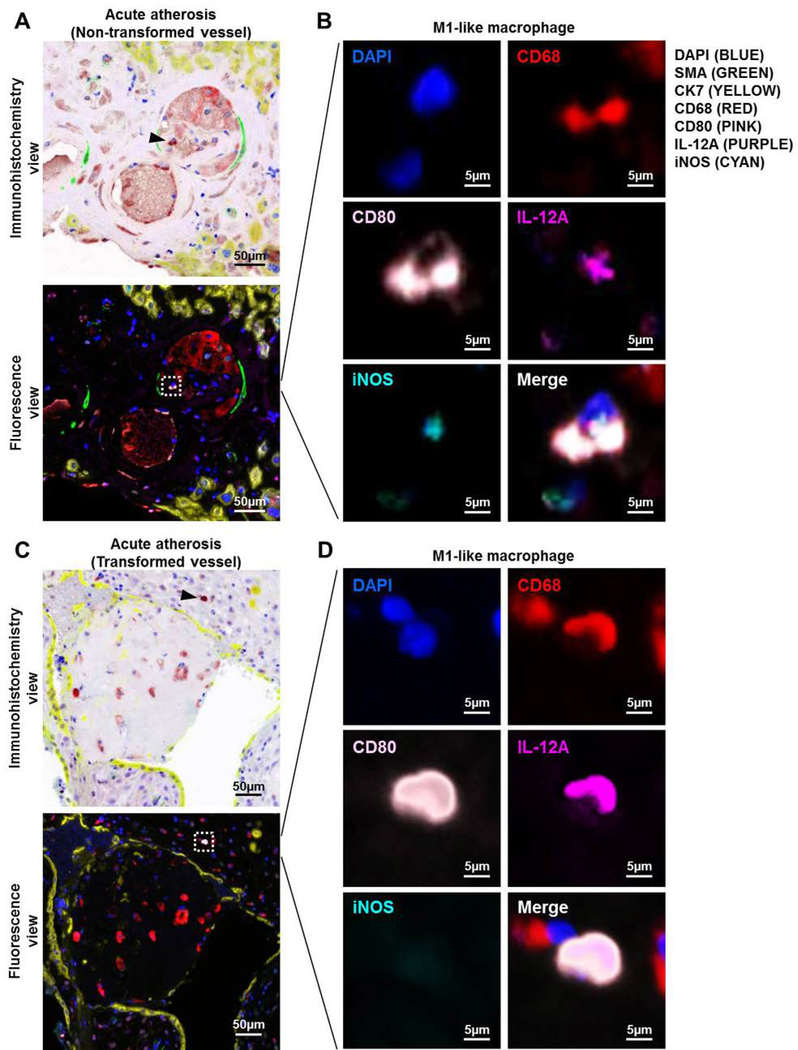
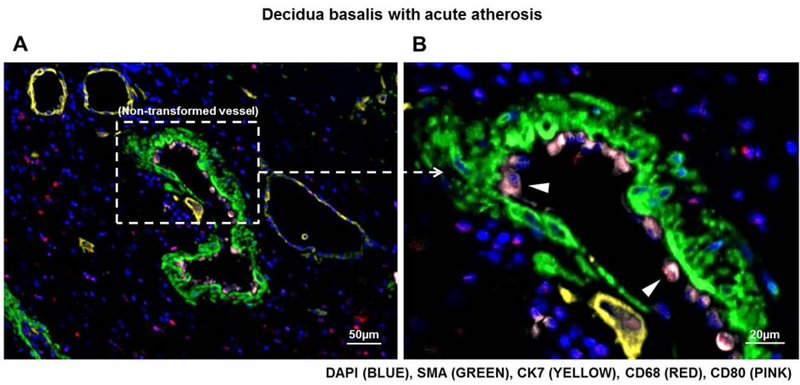
Non-transformed vessels are characterized by a lack of trophoblast, which invade the spiral arteries and contribute to transformation80, and the presence of smooth muscle cells, which are normally displaced or destroyed by invading trophoblast 2, 11. Figure 5A shows a non-transformed vessel, indicated by expression of smooth muscle actin (SMA) around the vessel and a lack of invading cytokeratin-7 (CK7)-positive trophoblast, in the decidua basalis with acute atherosis. Having identified non-transformed decidual vessels, we next determined whether M1-like macrophages were localized in these lesions. Macrophages expressing M1 markers (CD68+CD80+iNOS+IL-12+ cells) were detected in close proximity to non-transformed vessels in the decidua basalis with acute atherosis (Figure 5A, white dotted rectangle). A magnification of the mediators expressed by M1-like macrophages in non-transformed vessels with acute atherosis is shown in Figure 5B.
Figure 5. Immunolocalization of M1-like macrophages in the decidual vessels with acute atherosis.
Multiplex immunofluorescence staining showing nuclear staining (4′,6-diamidino-2-phenylindole, DAPI, blue), smooth muscle actin (SMA, green), cytokeratin-7 (CK7, yellow), CD68 (red), CD80 (pink), interleukin (IL)-12A (purple), and inducible nitric oxide synthase (iNOS, cyan) in the decidua basalis with acute atherosis. Representative images showing (A) the immunohistochemistry and fluorescence views and (B) magnification of an M1-like macrophage in the vessel wall of a non-transformed decidual vessel with acute atherosis. Representative images showing (C) the immunohistochemistry and fluorescence views and (D) magnification of an M1-like macrophage near a transformed decidual vessel with acute atherosis. Phenoptics was performed to generate separate and merged immunofluorescence images (B&D), and to convert fluorescence images to the immunohistochemistry view (A&C). Black arrows in the immunohistochemistry view and dotted boxes in the fluorescence view indicate an M1-like macrophage. Images are representative of 3 experiments per group. Images were taken at 200X magnification, and a close-up of an M1-like macrophage is shown. Scale bars = 50μm (original image) or 5μm (close-up image).
We then determined whether M1-like macrophages could be localized near transformed vessels in the decidua basalis as well. We identified transformed vessels in the decidua basalis with acute atherosis based on the presence of invading trophoblasts and lack of smooth muscle cells as previously described2, 11. We found that M1-like macrophages could be detected surrounding the transformed vessels in the decidua basalis with acute atherosis (Figures 5C, white dotted rectangle). A magnification of the mediators expressed by M1-like macrophages in transformed vessels with acute atherosis is shown in Figure 5D. These results demonstrate that M1-like macrophages are localized to both transformed and non-transformed vessels in the decidua basalis with acute atherosis.
Monocyte-like cells are present on the endothelium of non-transformed vessels: A possible intravascular origin for decidual macrophages with acute atherosis
Macrophages infiltrating atherosclerotic lesions are recruited from the intravascular space81. To complement our study, we addressed whether decidual macrophages in atherotic lesions could be infiltrating from the intravascular space. Interestingly, an accumulation of CD68-CD80+ monocyte-like cells was observed on the endothelium of non-transformed decidual vessels with acute atherosis (Figure 6A, white dotted box). A magnification of this image shows monocyte-like cells lining the vessel wall (Figure 6B). This finding suggests an intravascular origin for the decidual M1-like macrophages associated with lesions of acute atherosis.
Figure 6. Monocyte-like cells are present on the endothelium of non-transformed vessels with acute atherosis.
Multiplex immunofluorescence staining showing nuclear staining (4′,6-diamidino-2-phenylindole, DAPI, blue), smooth muscle actin (SMA, green), cytokeratin-7 (CK7, yellow), CD68 (red), and CD80 (pink) in the decidua basalis with acute atherosis. Representative and magnified images showing monocyte-like cells (CD68-CD80+ cells) localized on the endothelium of a non-transformed decidual vessel with acute atherosis. White arrows indicate monocyte-like cells. Phenoptics was performed to generate immunofluorescence images. Images are representative of 3 experiments per group. Images were taken at 200X magnification, and a close-up of monocyte-like cells on the vessel endothelium is shown. Scale bars = 50μm (original image) or 20μm (close-up image).
Acute atherosis is associated with the infiltration of MOX and Mhem macrophages in the decidua basalis
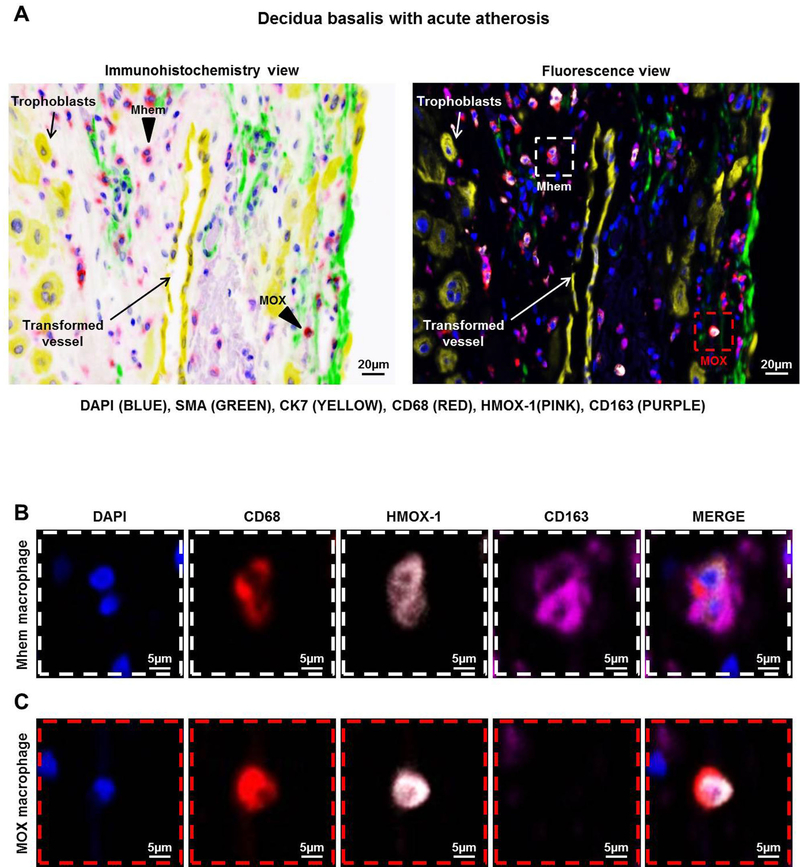
Given that a large proportion of decidual macrophages did not display an M1- or M2-like phenotype, we investigated the non-M1/M2 macrophage subsets present in the decidua basalis. MOX (CD68+HMOX-1+CD163- cells) and Mhem (CD68+HMOX-1+CD163+ cells) macrophages are present in atherosclerotic plaques82; therefore, we explored whether these macrophage populations were present in atherotic lesions using phenoptics. Both MOX and Mhem macrophages were present near transformed vessels (SMA-CK7+ vessels) in the decidua basalis with acute atherosis (Figure 7A, white or red dotted rectangles). A magnification of a Mhem macrophage expressing CD68, HMOX-1, and CD163 is shown in Figure 7B. A magnification of a MOX macrophage expressing CD68 and HMOX-1, but not CD163, is shown in Figure 7C. Together, these data indicate that, besides M1-like macrophages, MOX and Mhem macrophages are present in the decidua basalis with acute atherosis.
Figure 7. Acute atherosis is associated with HMOX-1-expressing decidual macrophage subsets.
Multiplex immunofluorescence staining showing nuclear staining (4′,6-diamidino-2-phenylindole, DAPI, blue), smooth muscle actin (SMA, green), cytokeratin-7 (CK7, yellow), CD68 (red), heme oxygenase-1 (HMOX-1, pink), and CD163 (purple) in the decidua basalis with acute atherosis. (A) Representative images showing the immunohistochemistry and fluorescence views of Mhem (CD68+HMOX-1+CD163+) (white dotted box) and MOX (CD68+HMOX-1+CD163-) (red dotted box) macrophages localized near a transformed decidual vessel with acute atherosis. Magnification of (B) a Mhem macrophage and (C) a MOX macrophage. Phenoptics was performed to generate separate and merged immunofluorescence images. Images are representative of 3 experiments per group. Images were taken at 200X magnification, and a close-up of a Mhem and MOX macrophage is shown. Scale bars = 20μm (original image) or 5μm (close-up image).
DISCUSSION
The principle findings of the study are as follows: 1) Macrophages displayed diverse phenotypes in the decidua basalis with acute atherosis; 2) decidual M1-like macrophages were increased in acute atherosis; 3) such decidual macrophages displayed a pro-inflammatory phenotype, as indicated by the expression of iNOS and IL-12; 4) M2-like macrophages were the most abundant subset in the decidua, yet this population did not change with the presence of acute atherosis; 5) decidual M1-like pro-inflammatory macrophages were localized near both transformed and non-transformed vessels with acute atherosis; 6) monocyte-like cells were present on the lumen of non-transformed vessels in the decidua basalis with acute atherosis, indicating a possible intravascular source for decidual macrophages; and 7) MOX and Mhem macrophages were identified near transformed vessels in the decidua basalis with acute atherosis.
M1- and M2-like decidual macrophages in acute atherosis
Macrophages represent a primary immune cell population in the decidua83–86. During early and mid-pregnancy, macrophages displaying an alternative or M2-like phenotype34, 36, 38, 40, 61 are the dominant decidual subset34, 36, 38, 58, indicating that such cells may play an important role in maternal-fetal tolerance throughout pregnancy34, 36, 37, 39, 40, 58. At the end of gestation, during the physiological process of labor, decidual macrophages acquire an M1-like phenotype58, a phenomenon that also occurs during the pathological process of preterm labor58 and coincides with the infiltration of other inflammatory cells at the maternal-fetal interface87–91. Disruption of the decidual macrophage population has been linked to placental dysfunction implicated in preeclampsia92, 93. Of interest, both women with spontaneous preterm labor94 and those with preeclampsia4, 95, 96 have a higher incidence of inadequate spiral artery remodeling, a condition often associated with the presence of acute atherosis6–11.
In the current study, we showed that decidual macrophages are a heterogeneous population comprised of M1- and M2-like macrophages as well as other subsets. M2-like macrophages are the most abundant decidual subset; yet, greater numbers of M1-like macrophages are found in the decidua basalis with acute atherosis compared to decidual tissues without this arterial lesion. Such macrophages expressed greater amounts of the pro-inflammatory mediator iNOS. Inducible NOS-deficient mice have a decreased incidence of diet-induced atherosclerosis and reduced plasma concentrations of lipid peroxides97. Together with studies showing that iNOS is expressed in human atherosclerotic plaques98–100, these findings suggest that decidual macrophages expressing iNOS participate in the pathophysiology of acute atherosis.
Macrophages also expressed high levels of IL-12 in the decidua basalis with acute atherosis. IL-12 is expressed in atherosclerotic plaques and is secreted by monocytes in response to oxidized low-density lipoproteins101. Indeed, the blockade of IL-12 signaling in a mouse model of atherosclerosis reduced atherogenesis and improved plaque stability102. These data indicate that, similar to atherosclerosis, IL-12 may be implicated in the inflammatory milieu which accompanies acute atherosis.
Interestingly, we showed that M1-like pro-inflammatory macrophages could be found near both non-transformed and transformed vessels in the decidua basalis with acute atherosis. Acute atherosis is a lesion that does not affect all of the decidual spiral arteries or every segment of such vessels11. Together, these data imply that the vascular-based inflammatory response characteristic of acute atherosis11, 21, 30, 103, 104 likely affects the entire decidua, inducing a tissue-wide M1 macrophage polarization.
A putative intravascular origin for decidual macrophages in acute atherosis
An intravascular inflammatory response can result in the activation of endothelial cells105–110 and accumulation of circulating monocytes103. During the pathogenesis of acute atherosis, monocytes seem to infiltrate the smooth muscle layer of affected spiral arteries30. As the tissue beneath the vessel endothelium becomes necrotic, it has been proposed that lipids released from the cellular membranes are ingested by scavenging macrophages leading to the formation of foam cells30. Herein, we observed monocyte-like cells on the endothelium of a non-transformed decidual vessel, suggesting that such cells extravasate from the intravascular space as an early step in the formation of acute atherotic lesions. This process resembles a similar mechanism for monocyte recruitment described in vessels with atherosclerosis81.
MOX and Mhem decidual macrophages in acute atherosis
Besides M1- and M2-like phenotypes, we also found macrophages expressing heme oxygenase-1 (HMOX-1) in the decidual tissues with acute atherosis. HMOX-1 is an enzyme which catalyzes the degradation of heme111 into carbon monoxide112, ferrous iron113, and biliverdin114. Such byproducts have antioxidant and anti-inflammatory properties and, therefore, HMOX-1 is considered an atheroprotective molecule115, 116. Yet, HMOX-1 expression has been described on two distinct macrophage subsets82: 1) “Mhem” macrophages, which are atheroprotective cells induced by CD163-mediated scavenging of hemoglobin in a heme-rich environment characteristic of atherosclerotic lesions117; and 2) “MOX” macrophages, which are inflammatory cells that lack CD163 expression and display reduced phagocytic capabilities in a microenvironment rich in oxidized phospholipids118. In the study herein, both MOX and Mhem macrophages were identified in the decidua basalis with acute atherosis. An imbalance between atherogenic and atheroprotective macrophage subsets has been implicated in the pathogenesis of atherosclerosis82. Moreover, acute atherosis has been linked to increased oxidative stress and lipid content in the decidua of preeclamptic women65, 119–121. Oxidative stress is also implicated in the pathophysiology of preeclampsia122–128 and fetal growth restriction129–131. Hence, we proposed that macrophages are implicated in the mechanisms leading to oxidative stress, and therefore, obstetrical disease (i.e. acute atherosis).
Conclusion
The current study shows that different subsets of macrophages are present in the decidua with acute atherosis, namely M1-like, M2-like, MOX, and Mhem subsets. Yet, these decidual lesions are enriched with M1-like macrophages that display a pro-inflammatory phenotype, as indicated by the expression of iNOS and IL-12. The fact that M1-like macrophages were present in both non-transformed and transformed vessels indicates that acute atherosis exerts a decidua-wide effect. Finally, we proposed an intravascular origin for decidual macrophages in acute atherosis. Collectively, these findings provide the first molecular characterization of the immunophenotype of decidual macrophages in acute atherosis.
Supplementary Material
Acknowledgements:
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit, as well as the research assistants from the PRB Clinical Laboratory, for their help in collecting samples.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Brosens I, Robertson WB, Dixon HG: The physiological response of the vessels of the placental bed to normal pregnancy. The Journal of pathology and bacteriology 1967;93:569–579. [DOI] [PubMed] [Google Scholar]
- 2.Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, Gotsch F, Than NG, Papp Z, Jai Kim C: Normal and abnormal transformation of the spiral arteries during pregnancy. Journal of perinatal medicine 2006;34:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton GJ, Jauniaux E: What is the placenta? Am J Obstet Gynecol 2015;213:S6 e1, S6–8. [DOI] [PubMed] [Google Scholar]
- 4.Brosens I, Pijnenborg R, Vercruysse L, Romero R: The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American journal of obstetrics and gynecology 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher SJ: Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 2015;213:S115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sexton LI, Hertig AT, Reid DE, Kellogg MD, Patterson WS: Premature separation of the normally implanted placenta; a clinicopathological study of 476 cases. American journal of obstetrics and gynecology 1950;59:13–24. [DOI] [PubMed] [Google Scholar]
- 7.Robertson WB, Brosens I, Dixon HG: The pathological response of the vessels of the placental bed to hypertensive pregnancy. The Journal of pathology and bacteriology 1967;93:581–592. [DOI] [PubMed] [Google Scholar]
- 8.De Wolf F, Robertson WB, Brosens I: The ultrastructure of acute atherosis in hypertensive pregnancy. American journal of obstetrics and gynecology 1975;123:164–174. [DOI] [PubMed] [Google Scholar]
- 9.Robertson WB, Brosens I, Dixon G: Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspectives in nephrology and hypertension 1976;5:115–127. [PubMed] [Google Scholar]
- 10.Staff AC, Dechend R, Pijnenborg R: Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension 2010;56:1026–1034. [DOI] [PubMed] [Google Scholar]
- 11.Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, Haas DM, Kassab GS, Romero R: Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. American journal of obstetrics and gynecology 2017;216:287 e281–287 e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton GJ, Jauniaux E: Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 2018;218:S745–S761. [DOI] [PubMed] [Google Scholar]
- 13.Zeek PM, Assali NS: Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy. American journal of clinical pathology 1950;20:1099–1109. [DOI] [PubMed] [Google Scholar]
- 14.Hertig AT: Vascular pathology in the hypertensive albuminuric toxemias of pregnancy. Clinics 1945:602–614. [Google Scholar]
- 15.Sheppard BL, Bonnar J: The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. British journal of obstetrics and gynaecology 1976;83:948–959. [DOI] [PubMed] [Google Scholar]
- 16.Brosens I, Dixon HG, Robertson WB: Fetal growth retardation and the arteries of the placental bed. British journal of obstetrics and gynaecology 1977;84:656–663. [DOI] [PubMed] [Google Scholar]
- 17.De Wolf F, Brosens I, Renaer M: Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. British journal of obstetrics and gynaecology 1980;87:678–685. [DOI] [PubMed] [Google Scholar]
- 18.Kitzmiller JL, Watt N, Driscoll SG: Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. American journal of obstetrics and gynecology 1981;141:773–779. [DOI] [PubMed] [Google Scholar]
- 19.Labarrere C, Alonso J, Manni J, Domenichini E, Althabe O: Immunohistochemical findings in acute atherosis associated with intrauterine growth retardation. American journal of reproductive immunology and microbiology : AJRIM 1985;7:149–155. [DOI] [PubMed] [Google Scholar]
- 20.Althabe O, Labarrere C, Telenta M: Maternal vascular lesions in placentae of small-for-gestational-age infants. Placenta 1985;6:265–276. [DOI] [PubMed] [Google Scholar]
- 21.Labarrere CA: Acute atherosis. A histopathological hallmark of immune aggression? Placenta 1988;9:95–108. [DOI] [PubMed] [Google Scholar]
- 22.Frusca T, Morassi L, Pecorelli S, Grigolato P, Gastaldi A: Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. British journal of obstetrics and gynaecology 1989;96:835–839. [DOI] [PubMed] [Google Scholar]
- 23.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, Hassan SS, Yeo L, Korzeniewski SJ: The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. The Journal of Maternal-Fetal and Neonatal Medicine 2015;28:2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, Hassan SS, Yeo L, Korzeniewski SJ: Placental lesions associated with acute atherosis. The Journal of Maternal-Fetal and Neonatal Medicine 2015;28:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, Kim JS, Yoon BH, Hassan SS, Yeo L: Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. American journal of obstetrics and gynecology 2016;214:629 e621–629 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson WB, Brosens I, Dixon G: Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol 1975;5:47–65. [DOI] [PubMed] [Google Scholar]
- 27.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A: A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–674. [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Kim YM: Acute Atherosis of the Uterine Spiral Arteries: Clinicopathologic Implications. Journal of Pathology and Translational Medicine 2015;49:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alnaes-Katjavivi P, Lyall F, Roald B, Redman CW, Staff AC: Acute atherosis in vacuum suction biopsies of decidua basalis: An evidence based research definition. Placenta 2016;37:26–33. [DOI] [PubMed] [Google Scholar]
- 30.Katabuchi H, Yih S, Ohba T, Matsui K, Takahashi K, Takeya M, Okamura H: Characterization of macrophages in the decidual atherotic spiral artery with special reference to the cytology of foam cells. Medical electron microscopy : official journal of the Clinical Electron Microscopy Society of Japan 2003;36:253–262. [DOI] [PubMed] [Google Scholar]
- 31.Hunt JS, Manning LS, Wood GW: Macrophages in murine uterus are immunosuppressive. Cellular immunology 1984;85:499–510. [DOI] [PubMed] [Google Scholar]
- 32.Tawfik OW, Hunt JS, Wood GW: Partial characterization of uterine cells responsible for suppression of murine maternal anti-fetal immune responses. J Reprod Immunol 1986;9:213–224. [DOI] [PubMed] [Google Scholar]
- 33.Repnik U, Tilburgs T, Roelen DL, van der Mast BJ, Kanhai HH, Scherjon S, Claas FH: Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta 2008;29:405–412. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J: Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 2008;3:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houser BL, Tilburgs T, Hill J, Nicotra ML, Strominger JL: Two unique human decidual macrophage populations. Journal of immunology 2011;186:2633–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J: Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. Journal of immunology 2011;187:3671–3682. [DOI] [PubMed] [Google Scholar]
- 37.Kim SY, Romero R, Tarca AL, Bhatti G, Kim CJ, Lee J, Elsey A, Than NG, Chaiworapongsa T, Hassan SS, Kang GH, Kim JS: Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. American journal of reproductive immunology 2012;68:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwan M, Hazan A, Zhang J, Jones RL, Harris LK, Whittle W, Keating S, Dunk CE, Lye SJ: Dynamic changes in maternal decidual leukocyte populations from first to second trimester gestation. Placenta 2014;35:1027–1034. [DOI] [PubMed] [Google Scholar]
- 39.Svensson-Arvelund J, Ernerudh J: The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. American journal of reproductive immunology 2015;74:100–109. [DOI] [PubMed] [Google Scholar]
- 40.Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC, Ernerudh J: The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. Journal of immunology 2015;194:1534–1544. [DOI] [PubMed] [Google Scholar]
- 41.Choudhury RH, Dunk CE, Lye SJ, Harris LK, Aplin JD, Jones RL: Decidual leucocytes infiltrating human spiral arterioles are rich source of matrix metalloproteinases and degrade extracellular matrix in vitro and in situ. Am J Reprod Immunol 2018:e13054. [DOI] [PubMed] [Google Scholar]
- 42.Mackaness GB: Cellular resistance to infection. J Exp Med 1962;116:381–406. [PubMed] [Google Scholar]
- 43.Nathan CF, Murray HW, Wiebe ME, Rubin BY: Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 1983;158:670–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celada A, Gray PW, Rinderknecht E, Schreiber RD: Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med 1984;160:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. Journal of immunology 2000;164:6166–6173. [DOI] [PubMed] [Google Scholar]
- 46.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH: Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A 2004;101:4560–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswas SK, Mantovani A: Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889–896. [DOI] [PubMed] [Google Scholar]
- 48.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H: Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010;70:4840–4849. [DOI] [PubMed] [Google Scholar]
- 49.Stein M, Keshav S, Harris N, Gordon S: Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992;176:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doyle AG, Herbein G, Montaner LJ, Minty AJ, Caput D, Ferrara P, Gordon S: Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol 1994;24:1441–1445. [DOI] [PubMed] [Google Scholar]
- 51.Anderson CF, Mosser DM: A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol 2002;72:101–106. [PubMed] [Google Scholar]
- 52.Mosser DM: The many faces of macrophage activation. J Leukoc Biol 2003;73:209–212. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology 2004;25:677–686. [DOI] [PubMed] [Google Scholar]
- 54.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA: Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 2009;5:e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon S, Martinez FO: Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 56.Biswas SK, Mantovani A: Orchestration of metabolism by macrophages. Cell Metab 2012;15:432–437. [DOI] [PubMed] [Google Scholar]
- 57.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M: Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of pathology 2013;229:176–185. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, Gomez-Lopez N: An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. Journal of immunology 2016;196:2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C: Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. American journal of reproductive immunology 2003;50:444–452. [DOI] [PubMed] [Google Scholar]
- 60.Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O: Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 2003;131:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cupurdija K, Azzola D, Hainz U, Gratchev A, Heitger A, Takikawa O, Goerdt S, Wintersteiger R, Dohr G, Sedlmayr P: Macrophages of human first trimester decidua express markers associated to alternative activation. American journal of reproductive immunology 2004;51:117–122. [DOI] [PubMed] [Google Scholar]
- 62.Laskarin G, Cupurdija K, Tokmadzic VS, Dorcic D, Dupor J, Juretic K, Strbo N, Crncic TB, Marchezi F, Allavena P, Mantovani A, Randic L, Rukavina D: The presence of functional mannose receptor on macrophages at the maternal-fetal interface. Hum Reprod 2005;20:1057–1066. [DOI] [PubMed] [Google Scholar]
- 63.Sheng YR, Hu WT, Wei CY, Tang LL, Liu YK, Liu YY, Qiu JP, Li DJ, Zhu XY: IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am J Reprod Immunol 2018;79:e12836. [DOI] [PubMed] [Google Scholar]
- 64.Zhao H, Kalish FS, Wong RJ, Stevenson DK: Hypoxia regulates placental angiogenesis via alternatively activated macrophages. Am J Reprod Immunol 2018;80:e12989. [DOI] [PubMed] [Google Scholar]
- 65.Staff AC, Halvorsen B, Ranheim T, Henriksen T: Elevated level of free 8-iso-prostaglandin F2alpha in the decidua basalis of women with preeclampsia. American journal of obstetrics and gynecology 1999;181:1211–1215. [DOI] [PubMed] [Google Scholar]
- 66.Staff AC, Dechend R, Redman CW: Review: Preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta 2013;34:13. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, Bhatti G, Kim JS, Qureshi F, Jacques SM, Jung EJ, Yeo L, Panaitescu B, Maymon E, Hassan SS, Hsu CD, Erez O: The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med 2018;46:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Plazyo O, Romero R, Hassan SS, Gomez-Lopez N: Isolation of Leukocytes from the Human Maternal-fetal Interface. J Vis Exp 2015:e52863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Villiers WJ, Smart EJ: Macrophage scavenger receptors and foam cell formation. J Leukoc Biol 1999;66:740–746. [DOI] [PubMed] [Google Scholar]
- 70.Micklem K, Rigney E, Cordell J, Simmons D, Stross P, Turley H, Seed B, Mason D: A human macrophage-associated antigen (CD68) detected by six different monoclonal antibodies. British journal of haematology 1989;73:6–11. [DOI] [PubMed] [Google Scholar]
- 71.Tsukamoto K, Kinoshita M, Kojima K, Mikuni Y, Kudo M, Mori M, Fujita M, Horie E, Shimazu N, Teramoto T: Synergically increased expression of CD36, CLA-1 and CD68, but not of SR-A and LOX-1, with the progression to foam cells from macrophages. Journal of atherosclerosis and thrombosis 2002;9:57–64. [DOI] [PubMed] [Google Scholar]
- 72.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A: Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 73.Edwards JP, Zhang X, Frauwirth KA, Mosser DM: Biochemical and functional characterization of three activated macrophage populations. Journal of Leukocyte Biology 2006;80:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA: Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Z, Jensen SM, Messenheimer DJ, Farhad M, Neuberger M, Bifulco CB, Fox BA: Multispectral Imaging of T and B Cells in Murine Spleen and Tumor. Journal of immunology 2016;196:3943–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang F, Ma Z, Pollan S, Yuan X, Swartwood S, Gertych A, Rodriguez M, Mallick J, Bhele S, Guindi M, Dhall D, Walts AE, Bose S, de Peralta Venturina M, Marchevsky AM, Luthringer DJ, Feller SM, Berman B, Freeman MR, Alvord WG, Vande Woude G, Amin MB, Knudsen BS: Quantitative imaging for development of companion diagnostics to drugs targeting HGF/MET. The journal of pathology Clinical research 2016;2:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parra ER, Uraoka N, Jiang M, Cook P, Gibbons D, Forget MA, Bernatchez C, Haymaker C, Wistuba II, Rodriguez-Canales J: Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Scientific reports 2017;7:13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorris MAJ, Halilovic A, Rabold K, van Duffelen A, Wickramasinghe IN, Verweij D, Wortel IMN, Textor JC, de Vries IJM, Figdor CG: Eight-Color Multiplex Immunohistochemistry for Simultaneous Detection of Multiple Immune Checkpoint Molecules within the Tumor Microenvironment. Journal of immunology 2018;200:347–354. [DOI] [PubMed] [Google Scholar]
- 79.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, Hsu CD: Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. American journal of reproductive immunology 2018:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pijnenborg R, Vercruysse L, Hanssens M: The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;27:939–958. [DOI] [PubMed] [Google Scholar]
- 81.Woollard KJ, Geissmann F: Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colin S, Chinetti-Gbaguidi G, Staels B: Macrophage phenotypes in atherosclerosis. Immunol Rev 2014;262:153–166. [DOI] [PubMed] [Google Scholar]
- 83.Bulmer JN, Johnson PM: Macrophage populations in the human placenta and amniochorion. Clinical and Experimental Immunology 1984;57:393–403. [PMC free article] [PubMed] [Google Scholar]
- 84.Starkey PM, Sargent IL, Redman CW: Cell populations in human early pregnancy decidua: characterization and isolation of large granular lymphocytes by flow cytometry. Immunology 1988;65:129–134. [PMC free article] [PubMed] [Google Scholar]
- 85.Lessin DL, Hunt JS, King CR, Wood GW: Antigen expression by cells near the maternal-fetal interface. American journal of reproductive immunology and microbiology : AJRIM 1988;16:1–7. [DOI] [PubMed] [Google Scholar]
- 86.King A, Wellings V, Gardner L, Loke YW: Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Human immunology 1989;24:195–205. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M: Immune cells in term and preterm labor. Cell Mol Immunol 2014;11:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N: An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol 2016;13:462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, Drewlo S, Tarca AL, Hassan SS, Gomez-Lopez N: Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol 2016;196:1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Schwenkel G, St Louis D, Hassan SS, Mial TN: In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin Exp Immunol 2017;189:211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS, Gomez-Lopez N: Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol 2018;79:e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faas MM, Spaans F, De Vos P: Monocytes and macrophages in pregnancy and pre-eclampsia. Frontiers in immunology 2014;5:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shimada S, Ebina Y, Iijima N, Deguchi M, Yamada H: Decidual CD68(+) HLA-DR(+) CD163(−) M1 macrophages increase in miscarriages with normal fetal chromosome. Am J Reprod Immunol 2018;79. [DOI] [PubMed] [Google Scholar]
- 94.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R: Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology 2003;189:1063–1069. [DOI] [PubMed] [Google Scholar]
- 95.Brosens IA, Robertson WB, Dixon HG: The role of the spiral arteries in the pathogenesis of preeclampsia. Obstetrics and gynecology annual 1972;1:177–191. [PubMed] [Google Scholar]
- 96.Khong TY, De Wolf F, Robertson WB, Brosens I: Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British journal of obstetrics and gynaecology 1986;93:1049–1059. [DOI] [PubMed] [Google Scholar]
- 97.Kuhlencordt PJ, Chen J, Han F, Astern J, Huang PL: Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 2001;103:3099–3104. [DOI] [PubMed] [Google Scholar]
- 98.Depre C, Havaux X, Renkin J, Vanoverschelde JL, Wijns W: Expression of inducible nitric oxide synthase in human coronary atherosclerotic plaque. Cardiovascular research 1999;41:465–472. [DOI] [PubMed] [Google Scholar]
- 99.Wilcox JN, Subramanian RR, Sundell CL, Tracey WR, Pollock JS, Harrison DG, Marsden PA: Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arteriosclerosis, thrombosis, and vascular biology 1997;17:2479–2488. [DOI] [PubMed] [Google Scholar]
- 100.Baker CS, Hall RJ, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM: Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arteriosclerosis, thrombosis, and vascular biology 1999;19:646–655. [DOI] [PubMed] [Google Scholar]
- 101.Uyemura K, Demer LL, Castle SC, Jullien D, Berliner JA, Gately MK, Warrier RR, Pham N, Fogelman AM, Modlin RL: Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. The Journal of clinical investigation 1996;97:2130–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hauer AD, Uyttenhove C, de Vos P, Stroobant V, Renauld JC, van Berkel TJ, van Snick J, Kuiper J: Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation 2005;112:1054–1062. [DOI] [PubMed] [Google Scholar]
- 103.Staff AC, Johnsen GM, Dechend R, Redman CWG: Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol 2014;101–102: [DOI] [PubMed] [Google Scholar]
- 104.Johnsen GM, Storvold GL, Drabbels JJM, Haasnoot GW, Eikmans M, Spruyt-Gerritse MJ, Alnaes-Katjavivi P, Scherjon SA, Redman CWG, Claas FHJ, Staff AC: The combination of maternal KIR-B and fetal HLA-C2 is associated with decidua basalis acute atherosis in pregnancies with preeclampsia. Journal of reproductive immunology 2018;129:23–29. [DOI] [PubMed] [Google Scholar]
- 105.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK: Preeclampsia: an endothelial cell disorder. American journal of obstetrics and gynecology 1989;161:1200–1204. [DOI] [PubMed] [Google Scholar]
- 106.Roberts JM, Edep ME, Goldfien A, Taylor RN: Sera from preeclamptic women specifically activate human umbilical vein endothelial cells in vitro: morphological and biochemical evidence. American journal of reproductive immunology 1992;27:101–108. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Zhang Y, Lewis DF, Gu Y, Li H, Granger DN, Alexander JS: Protease chymotrypsin mediates the endothelial expression of P- and E-selectin, but not ICAM and VCAM, induced by placental trophoblasts from pre-eclamptic pregnancies. Placenta 2003;24:851–861. [DOI] [PubMed] [Google Scholar]
- 108.Labarrere CA, Zaloga GP: C-reactive protein: from innocent bystander to pivotal mediator of atherosclerosis. The American journal of medicine 2004;117:499–507. [DOI] [PubMed] [Google Scholar]
- 109.Schnoor M, Alcaide P, Voisin MB, van Buul JD: Crossing the Vascular Wall: Common and Unique Mechanisms Exploited by Different Leukocyte Subsets during Extravasation. Mediators of inflammation 2015;2015:946509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerhardt T, Ley K: Monocyte trafficking across the vessel wall. Cardiovascular research 2015;107:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maines MD: The heme oxygenase system: a regulator of second messenger gases. Annual review of pharmacology and toxicology 1997;37:517–554. [DOI] [PubMed] [Google Scholar]
- 112.Maines MD: Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB Journal 1988;2:2557–2568. [PubMed] [Google Scholar]
- 113.Tenhunen R, Marver H, Pimstone NR, Trager WF, Cooper DY, Schmid R: Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry 1972;11:1716–1720. [DOI] [PubMed] [Google Scholar]
- 114.Tenhunen R, Marver HS, Schmid R: Microsomal heme oxygenase. Characterization of the enzyme. The Journal of biological chemistry 1969;244:6388–6394. [PubMed] [Google Scholar]
- 115.Abraham NG, Kappas A: Pharmacological and clinical aspects of heme oxygenase. Pharmacological reviews 2008;60:79–127. [DOI] [PubMed] [Google Scholar]
- 116.Ozen M, Zhao H, Kalish F, Yang Y, Folkins A, Burd I, Wong RJ, Stevenson DK: Heme oxygenase-1 deficiency results in splenic T-cell dysregulation in offspring of mothers exposed to late gestational inflammation. Am J Reprod Immunol 2018;79:e12829. [DOI] [PubMed] [Google Scholar]
- 117.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO: Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. American Journal of Pathology 2009;174:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N: Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 2010;107:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Staff AC, Ranheim T, Khoury J, Henriksen T: Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. American journal of obstetrics and gynecology 1999;180:587–592. [DOI] [PubMed] [Google Scholar]
- 120.Staff AC, Ranheim T, Halvorsen B: Augmented PLA2 activity in pre-eclamptic decidual tissue--a key player in the pathophysiology of ‘acute atherosis’ in pre-eclampsia? Placenta 2003;24:965–973. [DOI] [PubMed] [Google Scholar]
- 121.Harsem NK, Roald B, Braekke K, Staff AC: Acute atherosis in decidual tissue: not associated with systemic oxidative stress in preeclampsia. Placenta 2007;28:958–964. [DOI] [PubMed] [Google Scholar]
- 122.Redman CW, Sargent IL: Placental debris, oxidative stress and pre-eclampsia. Placenta 2000;21:597–602. [DOI] [PubMed] [Google Scholar]
- 123.McMaster-Fay RA: Oxidative stress and inflammatory biomarkers in normal and preeclamptic pregnancies. Am J Obstet Gynecol 2017;217:492–493. [DOI] [PubMed] [Google Scholar]
- 124.Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE: Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol 2017;216:527 e521–527 e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williamson RD, McCarthy C, McCarthy FP, Kenny LC: Oxidative stress in pre-eclampsia; have we been looking in the wrong place? Pregnancy Hypertens 2017;8:1–5. [DOI] [PubMed] [Google Scholar]
- 126.Romero R, Erez O, Huttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI: Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol 2017;217:282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bellos I, Karageorgiou V, Kapnias D, Karamanli KE, Siristatidis C: The role of interleukins in preeclampsia: A comprehensive review. Am J Reprod Immunol 2018:e13055. [DOI] [PubMed] [Google Scholar]
- 128.Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands JL: Oxidative stress in placental pathology. Placenta 2018;69:153–161. [DOI] [PubMed] [Google Scholar]
- 129.Lu L, Kingdom J, Burton GJ, Cindrova-Davies T: Placental Stem Villus Arterial Remodeling Associated with Reduced Hydrogen Sulfide Synthesis Contributes to Human Fetal Growth Restriction. Am J Pathol 2017;187:908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF: Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am J Reprod Immunol 2018;80:e13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sultana Z, Maiti K, Dedman L, Smith R: Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol 2018;218:S762–S773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.