ABSTRACT
The efficacy of nab-paclitaxel combined with gemcitabine (GnP) and of chemoradiotherapy (CRT) for unresectable locally advanced pancreatic ductal adenocarcinoma (UR-LA PDAC) is still unclear. We previously conducted a phase I study of CRT using GnP and determined the recommended dose and have now designed a phase II trial to evaluate the efficacy of CRT incorporating GnP for UR-LA PDAC. Eligibility criteria are chemotherapy-naïve patients with UR-LA PDAC as defined by the NCCN guidelines version 2. 2016. Study patients will receive 100 mg/m2 nab-paclitaxel and 800 mg/m2 gemcitabine on Days 1, 8, and 15 per 4-week cycle with concurrent radiation therapy (total dose of 50.4 Gy in 28 fractions of 1.8 Gy per day, 5 days per week). Treatment will be continued until disease progression or surgery, which is to be performed only for patients in whom the disease is well-controlled at 8 months from beginning the protocol treatment. Primary endpoint is 2-year overall survival rate and co-primary endpoint is resection rate. Secondary endpoints are overall survival, progression free survival, time to treatment failure, response rate, disease control rate, early tumor shrinkage, depth of response, reduction of SUV-max on PET–CT, serum tumor markers, relative dose intensity, safety, and Quality of life. This study will show the efficacy and safety of chemoradiotherapy combined with GnP.
Key Words: II study, Gemcitabine, nab-paclitaxel, chemoradiation, unresectable locally advanced pancreatic cancer
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the seventh leading cause of cancer-related death worldwide.1 PDAC has increased in Japan during the past few years, and is the fourth largest cause of cancer-related mortality, with 31,866 deaths in 2015.2,3 PDAC is typically diagnosed at the advanced stage and the overall 5-year survival rate is extremely poor at 2%–7%.4 Thirty –40% of patients with PDAC present with unresectable locally advanced disease (UR-LA).5,6
Two recent phase III trials have shown that either a combination of oxaliplatin, irinotecan, fluorouracil and leucovorin (FOLFIRINOX) or a combination of nab-paclitaxel with gemcitabine (GnP) achieve superior survival than gemcitabine alone in patients with metastatic PDAC.7,8 This is currently the standard treatment regimen for patients with metastatic PDAC and is also recommended by the National Comprehensive Cancer Network (NCCN) guidelines for UR-LA PDAC.9
Several prospective studies have reported that chemoradiotherapy (CRT) is more effective than chemotherapy or radiotherapy alone in patients with UR-LA PDAC.10-13 However, there are other trial results that support chemotherapy alone rather than CRT,14,15 and the optimal treatment strategy for this disease entity remains controversial.
In a previous phase I study exploring CRT in patients with UR-LA PDAC with a fixed dose of 50.4 Gy (1.8Gy /28fr) and escalating doses of GnP (NUPAT 02 Trial), we established a recommended dose of 800 mg/m2 for gemcitabine and 100 mg/m2 for nab-paclitaxel.16 We now plan to conduct a phase II trial (NUPAT 05 trial) to evaluate the safety and efficacy of this regimen in patients with UR-LA PDAC.
METHODS
Objectives
The NUPAT 05 trial is a phase II trial to be conducted by Nagoya University Pancreatic Tumor Board study group with the aim of evaluating safety and efficacy of CRT incorporating GnP in patients with UR-LA PDAC as defined by the NCCN resectability criteria.9
Study endpoints
The primary endpoint is 2-year overall survival rate by intention to treat. Resection rate will be evaluated as a co-primary endpoint. Secondary endpoints are overall survival, disease-free survival, time to treatment failure, overall response rate according to radiologic Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1,17 disease control rate, reduction in serum concentrations of tumor markers including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and PDAC-associated antigen (DUPAN2), early tumor shrinkage (ETS; defined as a reduction > 20% of target lesions’ diameters within 8 weeks of starting treatment), depth of response (DpR; defined as percentage of maximal tumor shrinkage at the nadir diameter compared with baseline), change in SUVmax of the primary tumor on PET/CT, safety according to Common Terminology Criteria for Adverse Event 4.0,18 relative dose intensity (RDI), and Quality of life (QoL) as assessed by the Functional Assessment of Cancer Therapy (FACT) and Patient Neurotoxicity Questionnaire (PNQ). This study will be performed in accordance with the declaration of Helsinki. The protocol has been approved by the Institutional Review Board of Nagoya University. All patients will provide written informed consent before enrollment in the study. The study is registered at UMIN-CTR (UMIN000028116).
Eligibility criteria
The inclusion and exclusion criteria for the NUPAT 05 trial are summarized in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Unresectable locally advanced pancreatic cancer based on the resectability status of the NCCN guidelines
2. Age between 20 and 75 (at the time of informed consent) 3. Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 4. No previous antitumor treatment 5. Within 10 × 10 cm radiation field for the primary lesion and regional lymph node 6. Adequate biliary drainage if obstructive jaundice is present 7. No direct invasion into the gastrointestinal tract 8. No simultaneous cancer in the other organs 9. Adequate oral intake 10. Expected more than 3 months survival 11. Adequate hematologic and major organ functions defined as; leukocyte count ≥ 3,500/mm3 and < 12,000/mm3, neutrophil count ≥ 2,000/mm3, hemoglobin level ≥ 9.0 g/dL, platelet count ≥ 100,000/mm3, total bilirubin level ≤ 2.0 mg/dL, aspartate aminotransferase and alanine aminotransferase levels ≤ 150 U/L, serum creatinine level ≤ 1.2 mg/dL, and creatinine clearance ≥ 50 mL/min 12. Written informed consent |
1. Distant metastasis
2. History of severe drug hypersensitivity or allergy 3. Invasion into gastrointestinal tract 4. Obvious infection or inflammation 5. Serious medical or psychological condition interfere with treatment 6. Other concurrent or previous malignancy within the past 5 years, except a curative carcinoma in situ or mucosal cancer 7. Peripheral neuropathy > grade 2 8. Ascites or pleural effusion 9. Active gastrointestinal bleeding required blood transfusion 10. Diarrhea > grade 2 11. Obvious fibroid lung or interstitial pneumonia 12. Previous abdominal radiotherapy 13. Uncontrolled severe cancer pain 14. Radiation field included lung 15. Pregnancy, or women who desire to have children 16. Any reason why, in the opinions of the investigator, the patient should not participate |
Statistical aspects
The primary endpoint of this study is the 2-year survival rate. In previous studies of patients with UR-LA PDAC, the 2-year survival rate after treatment with gemcitabine plus radiotherapy ranged from12% to15% and that after gemcitabine alone was 5%.12,13,19,20 In our department, CRT had also achieved better survival outcomes than chemotherapy alone (2-year survival rate: 47.1% vs. 19.1%). Additionally, better survival outcomes have been reported in patients with UR-LA PDAC receiving chemotherapy with GnP than in those receiving gemcitabine alone.9 We therefore hypothesized that, in patients with UR-LA PDAC, concurrent radiotherapy and GnP would achieve better survival outcomes than chemotherapy alone and assumed a 30% improvement in 2-year overall survival rate after CRT. We calculated that to prove an improvement in 2-year overall survival rate from 15% to 45% in a binominal test with alpha error of 0.05 and beta (power) of 0.817, assuming dropouts, a sample size of 25 patients was required. Resection rate will be also analyzed as co-primary endpoint.
Treatment
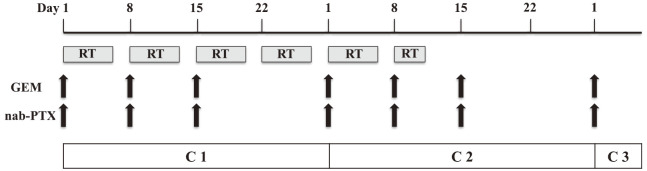
The study patients will receive 100 mg/m2 nab-paclitaxel intravenously over 30 min followed by 800 mg/m2 gemcitabine intravenously over 30 min. A total of 50.4Gy (1.8Gy/28fr) will be administered concurrently. GnP will be administered on Day 1, 8, and 15 per 4-week cycle. After two cycles of GnP have been administered, additional cycles will be continued until disease progression, an unacceptable level of adverse event(s), patient withdrawal, or conversion surgery (Fig. 1).
Fig. 1.
Treatment schedule.
RT; radiation therapy, GEM; gemcitabine, nab-PTX; nab-paclitaxel.
Conversion surgery (CS) may be considered in patients with stable disease or better, technically resectable tumors, normal tumor marker concentrations, decrease in SUVmax, and ECOG performance status of 0 or 1 8 months after starting the protocol treatment.
Follow-up
Follow-up will include physical examination, blood tests, and imaging. Tumor markers will be measured monthly and tumor responses evaluated according to RECIST version 1.117 using dynamic CT at 2 month intervals. PET/CT will be performed 3 monthly until disease progression or CS. Toxicities and adverse event will be evaluated in accordance with CTCAE version 4.0. Suspected unexpected life-threatening or fatal adverse events will be reported to our hospital’s director and Ministry of Health, Labor and Welfare. A data and safety monitoring board will monitor the trial subjects’ safety by qualitative assessment of feasibility, accrual rate, and toxicity/morbidity.
DISCUSSION
The NUPAT 05 trial will investigates the survival benefit of CRT combined with GnP in patients with UR-LA PDAC. Some clinical trials have shown that chemotherapy with GnP is effective against primary pancreatic tumors. Exploratory analysis of a phase 3 MPACT trial for metastatic PDAC indicated that reduction in primary pancreatic tumor burden is an independent predictor of survival.21 More recently, the phase 2 LAPACT trial showed that induction chemotherapy with GnP in patients with UR-LA PDAC was well tolerated and effective, the median PFS being 10.8 months.22 These results support the effectiveness of GnP in patients with UR-LA PDAC.
Of note, the CS rate was 15% in the LAPACT trial but 40%–50% in other studies using CRT combined with FOLFIRINOX in patients with UR-LA PDAC.23,24 In our phase 1 NUPAT 02 trial of CRT with GnP, CS was performed in 6/12 patients (50%) and curative resection (R0) achieved in all six (100%). Furthermore, we documented extremely good pathological responses (two grade 4, two grade 3, one grade 2 and one grade 1b). CS for UR-LA PDAC patients reportedly improves long-term survival.25 Some trials have also found an association between pathological response after CRT and long-term survival.26-28 Thus, we expect CRT incorporating GnP to improve resection rates and hence long-term survival and plan accordingly to evaluate resection rate statistically as a co-primary endpoint in this trial. A multicenter retrospective analysis of patients with UR-LA PDAC found that those who received chemo- or chemoradio-therapy for more than 8 months from diagnosis had better long-term survival.25 Therefore, in the planned trial, we will consider CS if a response with normalization of tumor marker concentrations is maintained 8 months after starting the protocol treatment.
Thus, both the efficacy and safety of CRT incorporating GnP and the impact of multidisciplinary treatment including CS on long-term survival of patients with UR-LA PDAC will be evaluated in this trial.
ACKNOWLEDGEMENT
This work is supported by Center for Medical and Clinical Research, Nagoya University Hospital, Japan. We are deeply grateful to the members in Nagoya University cancer board of pancreatic cancer. We also thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
DISCLOSURE STATEMENT
YA and YK received research funding and honoraria from Taiho Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd.. HG and TF received research funding and honoraria from Taiho Pharmaceutical Co., Ltd.
Abbreviations:
- PDAC
pancreatic ductal adenocarcinoma
- UR-LA
unresectable locally advanced disease
- CRT
chemoradiotherapy
- GnP
nab-paclitaxel with gemcitabine
- CS
conversion surgery
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87–108. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed]
- 2.Japanese Ministry of Health, Labor and Welfare. 2014.
- 3.Center for Cancer Control and Information Services NCC, Japan. http;//www.ncc.go.jp/en/cis.omdex.html. Accessed September 27, 2018.
- 4.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed]
- 5.Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33(16):1770–1778. doi: 10.1200/JCO.2014.59.7930 [DOI] [PubMed]
- 6.Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41(7):985–992. [DOI] [PubMed]
- 7.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed]
- 8.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed]
- 9.NCCN clinical practice guidelines in oncology (NCCN guidelines): NCCN; 2016
- 10.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48(8):1705–1710. [DOI] [PubMed]
- 11.Gastrointestinal Tumor Study Group. Treatment of locally unresectable cancer of the pancreas: composition of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80(10):751–755. [PubMed]
- 12.Loehrer PJ, Sr., Feng Y, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed]
- 13.Cardenes HR, Moore AM, Johnson CS, et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: a Hoosier Oncology Group study. Am J Clin Oncol. 2011;34(5):460–465. doi: 10.1097/COC.0b013e3181e9c103. [DOI] [PubMed]
- 14.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3(3):373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed]
- 15.Cohen SJ, Dobelbower R, Jr., Lipsitz S, et al. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62(5):1345–1350. doi: 10.1016/j.ijrobp.2004.12.074. [DOI] [PubMed]
- 16.Yamada S, Fujii T, Yokoyama Y, et al. Phase I study of chemoradiotherapy using gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2018;81(5):815–821. doi: 10.1007/s00280-018-3554-3. [DOI] [PubMed]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 2009;45:228–247. [DOI] [PubMed]
- 18.Common Terminology Criteria for Adverse Event 4.0. http//evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06.
- 19.Okusaka T, Ito Y, Ueno H, et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91(4):673–677. [DOI] [PMC free article] [PubMed]
- 20.Youl M, Hashem S, Brade A, et al. Induction gemcitabine plus concurrent gemcitabine and radiotherapy for locally advanced unresectable or resected pancreatic cancer. Clin Oncol (R Coll Radiol). 2014;26(4):203–209. doi: 10.1016/j.clon.2014.01.003. [DOI] [PubMed]
- 21.Kunzmann V, Ramanathan RK, Goldstein D, et al. Tumor reduction in primary and metastatic pancreatic cancer lesions with nab-paclitaxel and gemcitabine: an exploratory analysis from a phase 3 study. Pancreas. 2017;46(2):203–208. doi: 10.1097/MPA.0000000000000742. [DOI] [PMC free article] [PubMed]
- 22.Pascal H, Jill L, Fabienne P. Phase II LAPACT trial of nab-paclitaxel (nab-P) plus gemcitabine (G) for patients with locally advanced pancreatic cancer (LAPC). 2018. Gastrointestinal Cancer Symposium. (abstr 204).
- 23.Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, “unresectable” pancreatic cancer. Surgery. 2012;152(3 Suppl 1):S33–42. doi: 10.1016/j.surg.2012.05.029. [DOI] [PubMed]
- 24.Nanda RH, El-Rayes B, Maithel SK, et al. Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol. 2015;111(8):1028–1034. doi: 10.1002/jso.23921. [DOI] [PubMed]
- 25.Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013;20:590–600. [DOI] [PubMed]
- 26.Pietrasz D, Marthey L, Wagner M, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015;22(Suppl 3):S1196–1205. doi: 10.1245/s10434-015-4783-x. [DOI] [PubMed]
- 27.Chuong MD, Frakes JM, Figura N, et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol. 2016;7(2):221–227. doi: 10.3978/j.issn.2078-6891.2015.075. [DOI] [PMC free article] [PubMed]
- 28.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151(8):e161137. doi: 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed]