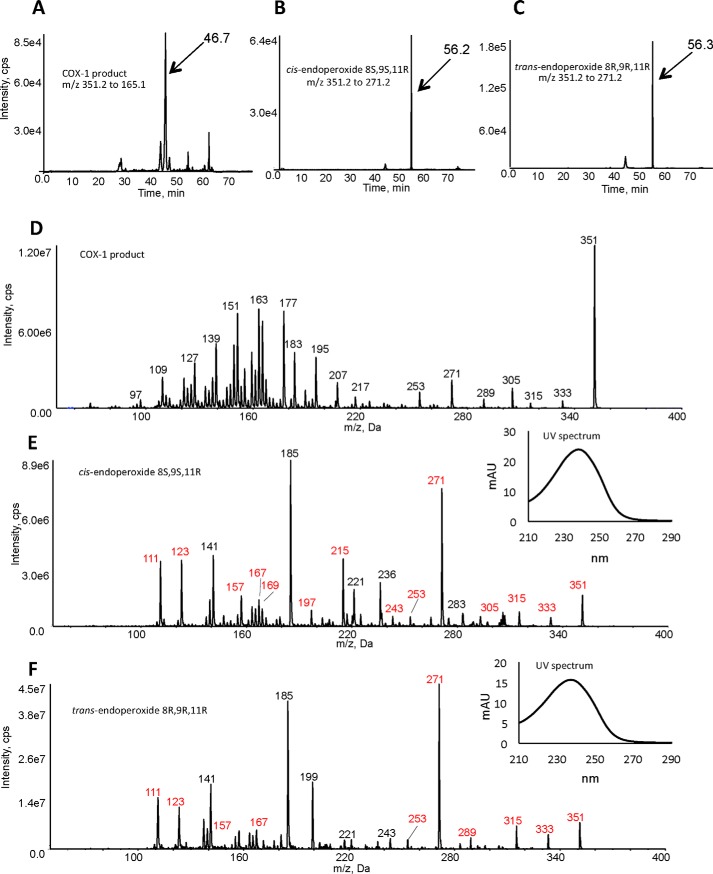
Figure 2.
Comparison of the COX-1–derived lipid with 8-hydroxy-9,11-dioxolanes indicates these are different lipids. A–C, reverse-phase LC-MS/MS indicates that the lipids elute at different retention times. Lipids (COX-1 product or 8-hydroxy-9,11-dioxolanes) were separated using reverse-phase LC-MS/MS as described under “Experimental procedures” and compared for retention time. Lipids were detected either as m/z 351.2 → 165.1 (COX-1 lipid) or 351.2 → 271.1 (dioxolanes). D--F, comparison of MS/MS spectra from the COX-1–derived lipid versus in vitro generated dioxolanes along with confirmation of conjugated diene structures for dioxolanes. MS/MS spectra were acquired at the peak of elution for the lipids shown in A–C above. Ions shown in red for E and F are common to the COX-1 product. Nominal mass is shown as these are low-resolution spectra (tandem quadrupole mass spectrometer). The inset UV spectra for the dioxolanes were obtained by HPLC-UV analysis as indicated under “Experimental procedures.” mAU, milli-absorbance units.