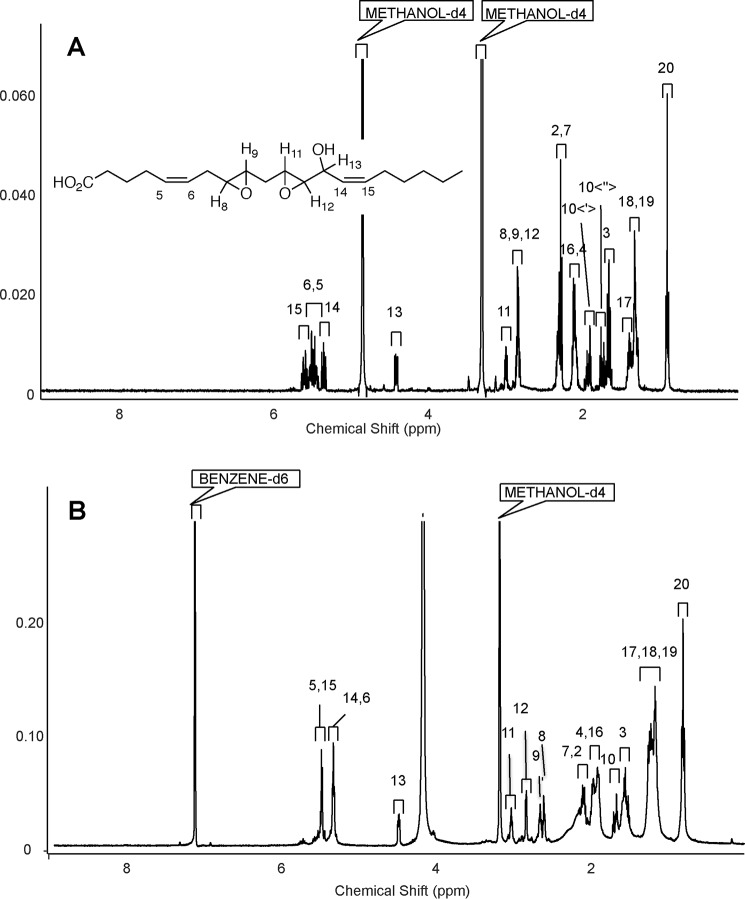
Figure 5.
1H NMR data of the synthetic lipid in methanol-d4 and benzene-d6 shows the structure as 8,9–11,12-DiEp-13-HEDE. A, 1H NMR (methanol-d4; 400 MHz) δ 5.60 (dtd, 1H, J = 1.17, 7.54, 11.08 Hz), 5.48 (m, 2H), 5.35 (tdd, 1H, J = 1.56, 8.59, 10.94 Hz), 4.41 (ddd, 1H, J = 1.17, 4.10, 8.79 Hz), 2.97–3.00 (m, 1H), 2.86–2.81 (m, 3H), 2.34–2.31 (m, 2H), 2.28 (t, 2H, 7.23 Hz), 2.13–2.07 (m, 4H), 1.95–1.89 (m, 1H), 1.77–1.71 (m, 1H), 1.66 (qint, 2H, 7.23 Hz), 1.41–1.36 (m, 2H), 1,31–1.28 (br m, 4H), 0.90 (t, 3H, 7.0 Hz). B, 1H NMR (benzene-d6:methanol-d4, 90:10; 700 MHz) δ 5.51–5.43 (m, 2H), 5.36–5.29 (m, 2H), 4.48 (dd, 1H, J = 7.75, 4.0 Hz), 3.03 (dt, 1H, J = 2.20, 5.1 Hz), 2.83 (dd, 1H, J = 2.13, 3.83 Hz), 2.65 (dt, 1H, J = 2.25, 5.20 Hz), 2.61 (dt, 1H, J = 2.21, 5.36 Hz), 2.14–2.05 (m, 2H), 2.02–1.85 (m, 4H), 1.72–1.63 (dt, 1H, J = 15.03, 4.58 Hz), 1.58–1.48 (m, 3H), 1.31–1.10 (m, 8H), 0.81 (t, 3H, J = 6.9 Hz).