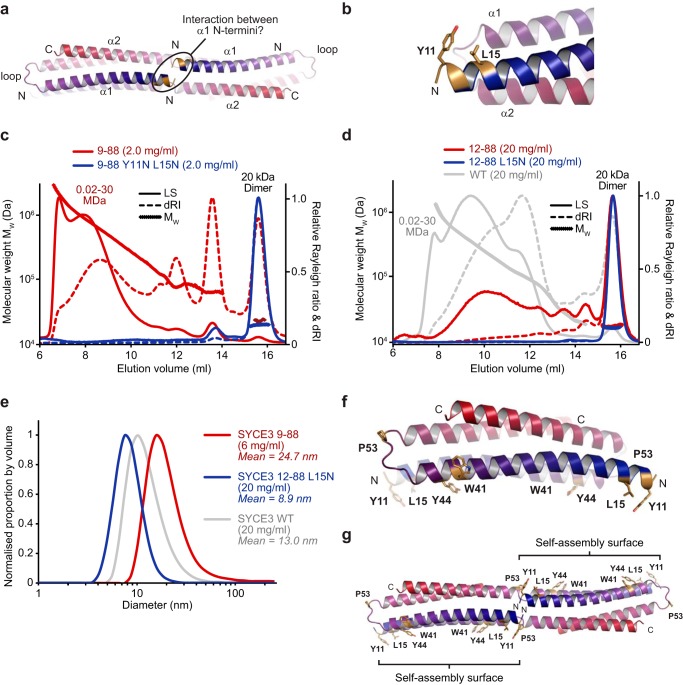
Figure 5.
SYCE3 self-assembly is facilitated by the N-terminal tip of the α1 helix. a, SYCE3 modeled domain-swap tetramer in which the N termini of capping chain α1 helices are highlighted, indicating their potential proximity and possible interaction in assembled structures. b, the N terminus of α1 helix includes amino acids Tyr-11 and Leu-15, which are solvent-exposed and do not contribute to the SYCE3 dimer fold. c and d, SEC-MALS analysis. c, SYCE3 9–88 (red) forms high-molecular weight assemblies, whereas 9–88 Y11N/L15N (blue) is largely restricted to a 20-kDa dimer (theoretical dimer, 21 kDa). d, SYCE3 12–88 (red) is mostly dimeric with some higher-order structures, whereas 12–88 L15N (blue) forms only a 20-kDa dimer (theoretical dimer, 21 kDa); WT is shown in gray for comparison. e, DLS analysis. SYCE3 9–88 (red) forms large particles of size greater than WT assemblies (gray), whereas 12–88 L15N (blue) forms only small particles of mean diameter 8.9 nm. f and g, summary of the molecular determinants of SYCE3 self-assembly. Shown are SYCE3 dimeric crystal structure (f) and modeled domain-swap tetramer (g) highlighting the positions of surface aromatic residues Trp-41 and Tyr-44, loop residue Pro-53, and α1 N-terminal residues Tyr-11 and Leu-15.