Figure 2.
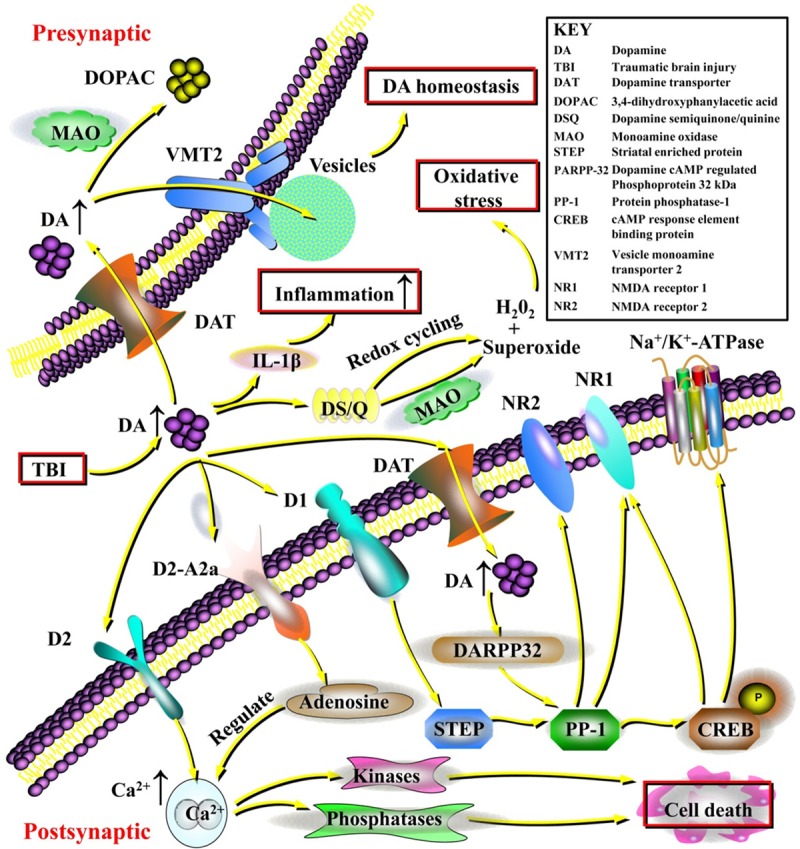
The contribution of dopamine to intracellular signaling and the essential neuroprotective effects of dopaminergic regulation following traumatic brain injury (TBI). Excessive dopamine could exert potent excitotoxic effects, inducing the generation of hydrogen peroxide (H2O2) and superoxide causing significant oxidative stress. In addition, dopamine can act as a potent inflammatory agent inducing inflammatory cytokines such as interleukin-1β (IL-1β), and dopaminergic regulation can potentially be important for reducing inflammation within the central nervous system (CNS). Excessive dopamine that is not metabolized by monoamine oxidase (MAO) could be recycled into vesicles by the vesicular monoamine transporter 2 (VMT2), maintaining dopamine homeostasis. Furthermore, the cellular mechanisms regarding the neuroprotective effects of dopamine in TBI have also been revealed. Dopamine exerts important effects on the regulation of Na+/K+-ATPase, Ca2+ release, and the N-methyl-D-aspartic acid receptor (NMDA) receptor through dopamine, cAMP-regulated phosphoprotein 32 kDa (DARPP-32) and protein phosphatase-1 (PP-1). Specifically, dopamine acting on D1 receptors can modify the activity of striatal enriched protein (STEP), which contributes to PP-1 activity. PP-1 also regulates nuclear transcription through cAMP response element-binding protein (CREB) phosphorylation and plays an important role in the phosphorylation of the NMDA NR1 subunit and the Na+/K+-ATPase. Dopamine signaling at the dopamine D2 receptor can induce increases in intracellular Ca2+ release and activation of calcium-dependent kinases and phosphatases, which is important for cell death signaling. Dopamine also forms a tight signaling relationship with adenosine via dopamine D2/adenosine A2a receptor interactions that can directly control intracellular Ca2+ release. Clarifying these dopaminergic signaling pathways could help identify promising therapies targeting dopamine and direct attention to this neurotransmitter for clinical applications.
