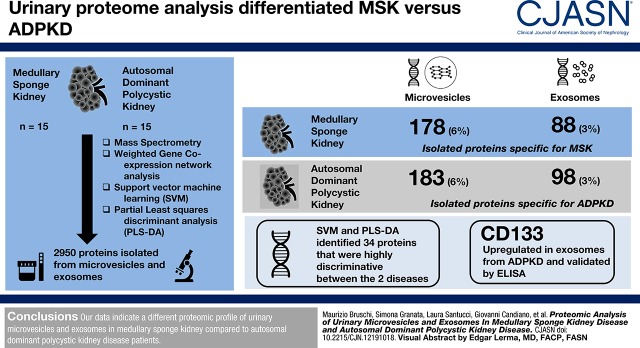
Visual Abstract
Keywords: Medullary Sponge Kidney; autosomal dominant polycystic kidney disease; proteomics; Polycystic Kidney, Autosomal Dominant; Nephrocalcinosis; Exosomes; calcium; Calcification, Physiologic; Proteomics; Discriminant Analysis; Least-Squares Analysis; Support Vector Machine; Flow Cytometry; Kidney Calculi; Cell-Derived Microparticles; Mass Spectrometry; Cysts; Enzyme-Linked Immunosorbent Assay; Cell Proliferation
Abstract
Background and objectives
Microvesicles and exosomes are involved in the pathogenesis of autosomal dominant polycystic kidney disease. However, it is unclear whether they also contribute to medullary sponge kidney, a sporadic kidney malformation featuring cysts, nephrocalcinosis, and recurrent kidney stones. We addressed this knowledge gap by comparative proteomic analysis.
Design, setting, participants, & measurements
The protein content of microvesicles and exosomes isolated from the urine of 15 patients with medullary sponge kidney and 15 patients with autosomal dominant polycystic kidney disease was determined by mass spectrometry followed by weighted gene coexpression network analysis, support vector machine learning, and partial least squares discriminant analysis to compare the profiles and select the most discriminative proteins. The proteomic data were verified by ELISA.
Results
A total of 2950 proteins were isolated from microvesicles and exosomes, including 1579 (54%) identified in all samples but only 178 (6%) and 88 (3%) specific for medullary sponge kidney microvesicles and exosomes, and 183 (6%) and 98 (3%) specific for autosomal dominant polycystic kidney disease microvesicles and exosomes, respectively. The weighted gene coexpression network analysis revealed ten modules comprising proteins with similar expression profiles. Support vector machine learning and partial least squares discriminant analysis identified 34 proteins that were highly discriminative between the diseases. Among these, CD133 was upregulated in exosomes from autosomal dominant polycystic kidney disease and validated by ELISA.
Conclusions
Our data indicate a different proteomic profile of urinary microvesicles and exosomes in patients with medullary sponge kidney compared with patients with autosomal dominant polycystic kidney disease. The urine proteomic profile of patients with autosomal dominant polycystic kidney disease was enriched of proteins involved in cell proliferation and matrix remodeling. Instead, proteins identified in patients with medullary sponge kidney were associated with parenchymal calcium deposition/nephrolithiasis and systemic metabolic derangements associated with stones formation and bone mineralization defects.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2019_04_24_CJASNPodcast_19_06_.mp3
Introduction
Extracellular vesicles, such as microvesicles (diameter of 100–1000 nm) and exosomes (diameter of 30–100 nm), are membrane-enclosed particles released by most cells under normal and pathologic conditions (1–5). Microvesicles are shed directly from the plasma membrane, whereas exosomes are formed by the fusion of intracellular multivesicular bodies (also known as late endosomes) with the plasma membrane, leading to the release of their vesicular contents into the extracellular space. These vesicles can mobilize a large number of biologic factors, including receptors, other proteins, nucleic acids, and lipids, thus shuttling information to other cells (6). The transfer of RNA and miRNA can reprogram recipient cells and modify their phenotype (7).
The hypothesis that extracellular vesicles are present in human urine (8) was confirmed by the proteomic identification of membrane proteins in a pellet isolated by the ultracentrifugation of urine samples (9). Such urinary extracellular vesicles contain cell-specific marker proteins from every segment of the nephron (9,10), and they offer a source of potentially valuable urinary biomarkers (10). The intrinsic characteristics of extracellular vesicles also suggest that they may play an important role in kidney development and kidney disease. Accordingly, extracellular vesicles seem to be involved in the mechanism of cystogenesis in autosomal polycystic kidney disease, a common hereditary kidney disorder with a prevalence of 0.1%–0.25%. Autosomal polycystic kidney disease gives rise to predominantly kidney symptoms, including cysts that progressively disrupt the kidney parenchyma, leading to interstitial fibrosis, cellular infiltration, and the loss of functional nephrons.
The proteomic analysis of urinary exosome-like vesicles (particularly those containing polycystin) revealed approximately 500 autosomal dominant polycystic kidney disease–associated proteins, many with signaling functions (11). Furthermore, the quantitative proteomic analysis of urinary extracellular vesicles from patients affected by a complete spectrum of chronic kidney functional damage highlighted 30 proteins strongly associated with the autosomal dominant polycystic kidney disease phenotype, including periplakin, envoplakin, villin-1, and complement C3 (12).
In contrast to the wealth of information available for autosomal dominant polycystic kidney disease, little is known about the role of extracellular vesicles in the onset of medullary sponge kidney, a sporadic cystic kidney malformation that involves nephrocalcinosis and recurrent kidney stones (13). The detailed analysis of extracellular vesicles could provide insight into the pathogenesis of this rare disease. Despite sporadic genetic associations (14,15) and the dysregulation of a few biologic factors (16–18), the systemic and kidney biologic/cellular network underlying this disease is poorly characterized, and its relationship with other cystic diseases is unclear.
To address this knowledge gap, we carried out a comprehensive comparative proteomic analysis of urinary microvesicles and exosomes to identify differences between medullary sponge kidney and autosomal dominant polycystic kidney disease in terms of the mechanism of cystogenesis and identify putative diagnostic biomarkers that distinguish these diseases. In fact, at the moment, no diagnostic biomarkers are available for both diseases. Although some urinary biomarkers for autosomal dominant polycystic kidney disease (NGAL, M-CSF, and MCP-1) (19) have been proposed, none of them have been used in clinical practice (19). Additionally, most of them are only effective in the advanced stage of the disease. Identification of both diseases at early stages could help clinicians start prevention, diet adjustment, and for selected patients, pharmacologic treatment. Finally, they could potentiate diagnostic accuracy for medullary sponge kidney (this disease is often undiagnosed and confused with other cause of nephrocalcinosis or papillary ductal plugging), minimize patients’ radiation and/or nephrotoxic contrast media exposure from medical imaging (e.g., intravenous urography and CT urography), and reduce underdiagnosis of noncontrast CT scans.
Materials and Methods
Patients
The study included 15 adult patients with autosomal dominant polycystic kidney disease and 15 adult patients with medullary sponge kidney matched for age, sex, and geographical origin as well as a cohort of 17 healthy donors matched for age and sex (Table 1, Supplemental Figure 1). The patients were followed up by the Renal Unit at the Department of Medicine, University Hospital of Verona (Verona, Italy), and they were enrolled after providing informed consent. Medullary sponge kidney diagnosis was performed as previously reported (15). The diagnosis of autosomal dominant polycystic kidney disease was dependent on the revised Ravine criteria (20). The study was carried out in accordance with the Declaration of Helsinki and approved by the institutional ethical board of the University Hospital of Verona (Verona, Italy; code 1312CESC) and the Independent Ethics Committee (Comitato Etico Regione Liguria) on October 14, 2014 (study number 408REG2014).
Table 1.
Baseline characteristics of the study participants
| Variable | Medullary Sponge Kidney | Autosomal Dominant Polycystic Kidney Disease | Healthy Controlsa |
|---|---|---|---|
| Age, yr | 26±4 | 26±5 | 27±8 |
| Sex (men/women) | 6/9 | 7/8 | 8/9 |
| eGFR, ml/min per 1.73 m2 | 132±15 | 133±12 | 139±8 |
| Plasma calcium, mg/dl | 9.5±0.3 | 9.4±0.4 | 9.4±0.3 |
| Plasma phosphate, mg/dl | 3.1±0.5 | 2.9±0.5 | 2.8±0.5 |
| Plasma sodium, mmol/L | 140±2 | 139±2 | 138±4 |
| Plasma potassium, mmol/L | 3.8±0.6 | 3.9±0.2 | 3.9±0.1 |
| Proteinuria, g/24 h | 0.08±0.06 | 0.07±0.07 | 0.04±0.09 |
| Urine volume, ml/d | 1786±212 | 1750±581 | 1742±526 |
| Systolic BP, mm Hg | 119±4 | 118±7 | 117±5 |
| Diastolic BP, mm Hg | 74±5 | 76±6 | 75±4 |
Values are expressed as mean±SD. P values were determined by ANOVA except for sex, which was determined by Fisher exact test.
Included only in the flow cytometry analysis.
Isolation of Microvesicles and Exosomes
Second morning urine samples were obtained from patients and healthy donors. Extracellular vesicles were isolated by centrifugation. Briefly, aliquots of 16 ml were centrifuged at 16,000×g for 30 minutes at 16°C to remove cells, debris, and organelles, such as mitochondria. To obtain the microvesicle fraction, the supernatant was centrifuged at 22,000×g for 120 minutes at 16°C (21). The microvesicle pellet was rinsed in PBS and centrifuged again at 22,000×g; this rinse/centrifugation cycle was carried out five times in total to obtain a clean microvesicle fraction. The supernatant was then centrifuged at 100,000×g for 120 minutes at 16°C to pellet the exosomes. The pellet was resuspended in 1 ml 0.25 M sucrose, loaded on a 1-ml 30% sucrose cushion, and centrifuged at 100,000×g for 120 minutes at 16°C. The pellet was rinsed in PBS and centrifuged again at 100,000×g for 10 minutes at 4°C, and this rinse/centrifugation cycle was carried out five times in total to obtain a clean exosome fraction. For each assay, we have performed the same purification procedure. Each pellet fraction was stored at −80°C until use. The size and purity of microvesicles and exosomes isolated by ultracentrifugation were confirmed by dynamic light scattering, whereas the antigen profile of exosomes and microvesicles was performed by Western blot as described in Supplemental Material.
Mass Spectrometry
The samples were processed by the in-StageTip method with two poly(styrene divinylbenzene) reverse phase sulfonate disks (22). Each pellet was solubilized in 25 μl 2% sodium deoxycholate, 10 mM Tris(2-carboxyethyl)phosphine, 40 mM chloroacetamide, and 100 mM Tris (pH 8.5). Microvesicles or exosomes were lysed, reduced, and alkylated in a single step, and then, they were loaded into the StageTip. The lysates were diluted with 25 mM Tris (pH 8.5) containing 1 μg of trypsin. The samples were acidified with 100 μl 1% (vol/vol) trifluoroacetic acid and washed three times with 0.2% (vol/vol) trifluoroacetic acid. The proteins were eluted in 60 μl 5% (vol/vol) ammonium hydroxide containing 80% (vol/vol) acetonitrile. Detailed descriptions of mass spectrometry instrumentation, data analysis, and biologic validation with homemade ELISA are reported in Supplemental Material.
Statistical Analyses
After normalization using the Normalyzer R-package with the LOESS-G method (23), mass spectrometry data were analyzed by unsupervised hierarchical clustering using multidimensional scaling with k means and Spearman correlation to identify outliers and the dissimilarity between samples. The normalized expression profiles of the proteins were then used to construct the coexpression network using the weighted gene coexpression network analysis package in R (24). Additionally, to identify the hub proteins of modules that maximize the discrimination between the selected clinical traits, we applied a nonparametric Mann–Whitney U test, machine learning methods (such as nonlinear support vector machine learning), and partial least squares discriminant analysis. A complete and detailed description of the data analysis has been reported in Supplemental Material.
Results
Characterization of Exosomes and Microvesicles
The size and purity of microvesicles and exosomes isolated by ultracentrifugation were confirmed by dynamic light scattering, revealing a Gaussian distribution profile with peak means at 1000±65 or 90±5 nm, respectively, the typical sizes for microvesicles or exosomes, respectively (Supplemental Figure 2, A and B). There was no difference in size between the microvesicles and exosomes isolated from patients with medullary sponge kidney and patients with autosomal dominant polycystic kidney disease. Western blot analysis revealed that the exosomes were positive for CD63 and CD81 but not CD45, whereas the microvesicles showed the opposite antigen profile (Supplemental Figure 2C).
Protein Composition of Exosomes and Microvesicles
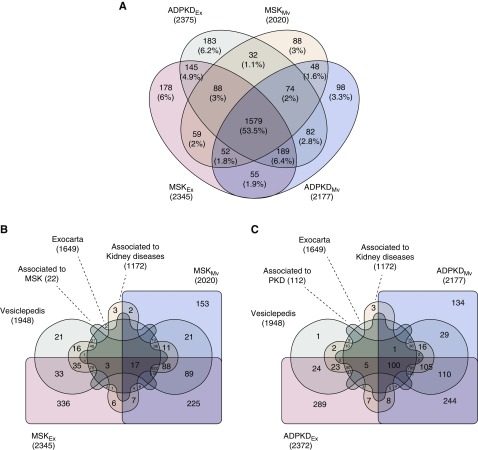
The protein composition of exosomes and microvesicles from the urine of patients with medullary sponge kidney and patients with autosomal dominant polycystic kidney disease was determined by mass spectrometry. We identified 2950 proteins in total, 1579 (54%) of which were present in all four sample types. Among the medullary sponge kidney samples, only 178 (6%) and 88 (3%) proteins were exclusively found in the exosomes and microvesicles, respectively. Similarly, among the autosomal dominant polycystic kidney disease samples, only 183 (6%) and 98 (3%) proteins were exclusively found in the exosomes and microvesicles, respectively (Figure 1A); >60% of all of the extracellular vesicle proteins that we identified were present in exosomes, and >80% were present in microvesicles.
Figure 1.
Venn diagram of total proteins detected in exosomes and microvesicles from the urine of patients with medullary sponge kidney (MSK) and patients with autosomal dominant polycystic kidney disease (ADPKD) identified by mass spectrometry. (A) The Venn diagram shows common and exclusive proteins in MSK and ADPKD. The numbers represent the distinct proteins in the overlapping and nonoverlapping areas. (B and C) The numbers represent the distinct proteins in the overlapping and nonoverlapping areas. The data were extracted from the Exocarta, Vesiclepedia, UniProt, Open Target, DisGeNET, and Atlas databases. The majority of the proteins identified in extracellular vesicles correspond to proteins already described as components of exosomes or microvesicles or associated with kidney disease (about 40%). We found that 95% and 100% of the proteins were associated with MSK and ADPKD, respectively. PKD, polycystic kidney disease.
Furthermore, about 40% of the proteins found in extracellular vesicles were associated with one or both kidney diseases: 95% were found in the medullary sponge kidney samples, and 100% were found in the autosomal dominant polycystic kidney disease samples (Figure 1, B and C). The cellular origins of the proteins in the exosomes were very similar in the medullary sponge kidney and autosomal dominant polycystic kidney disease samples, with 18% of proteins originating from membranes, 32% originating from the cytoplasm, 10% originating from the nucleus, and 39% originating from other organelles (Supplemental Figure 3). Similar results were observed for the microvesicle proteins, with 34% originating from membranes, 26% originating from the cytoplasm, 8% originating from the nucleus, and 32% originating from other organelles.
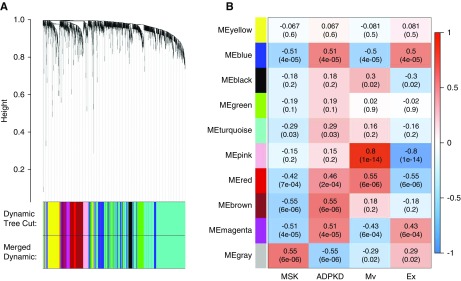
The significant overlap among the groups of proteins found in each sample was confirmed by constructing a two-dimensional scatter plot of the multidimensional scaling analysis (Supplemental Figure 4). No samples were excluded during the quality check performed by nonhierarchical clustering (Supplemental Figure 5). We used weighted gene coexpression network analysis to identify proteins associated with each type of extracellular vesicle and disease, revealing a total of ten modules comprising proteins with similar expression profiles. To distinguish between modules, we chose an arbitrary color for each module (Figure 2A). The number of proteins included in each module ranged from 44 (gray) to 930 (turquoise). The gray, brown, pink, and blue modules showed closer relationships with the medullary sponge kidney, autosomal dominant polycystic kidney disease, microvesicle, and exosome groups, respectively (Figure 2B).
Figure 2.
Module identification and clinical trait relationship. (A) Dendrogram of all proteins identified in the extracellular vesicles of patients with medullary sponge kidney (MSK) and patients with autosomal dominant polycystic kidney disease (ADPKD) clustered on the basis of a dissimilarity measure with topological overlap matrix (TOM) (1-TOM). (B) Heat map of the relationships between module eigengenes and the trait indicator of samples. Module-trait weighted relationships and their P values (in parentheses) between the identified modules and trait indicator. The color scale on the right shows module-trait relationship from −1 (blue) to one (red), where blue represents a perfect negative correlation and red represents a perfect positive correlation. Mv, microvesicles; Ex, exosomes; ME, module eigengenes.
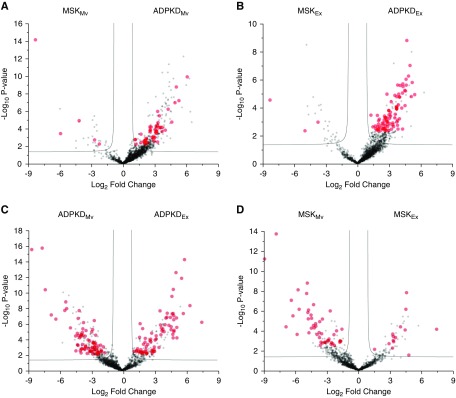
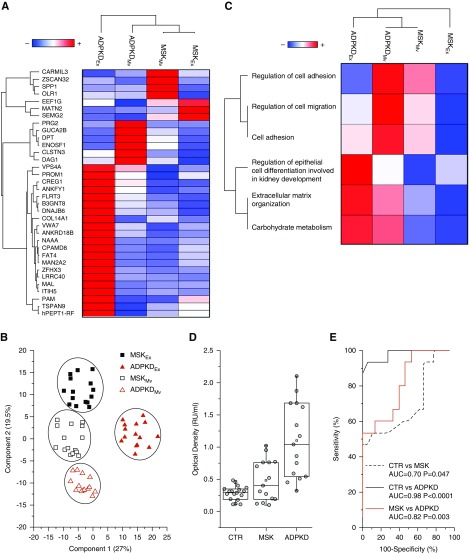
Next, we applied the Mann–Whitney U test to identify the proteins that best distinguish the type of disease in the microvesicles or exosomes (Figure 3, A and B) and the type of extracellular vesicle in the medullary sponge kidney or autosomal dominant polycystic kidney disease samples (Figure 3, C and D). This revealed a total of 255 discriminatory proteins, 50 that distinguished between medullary sponge kidney and autosomal dominant polycystic kidney disease microvesicles, 90 that distinguished between medullary sponge kidney and autosomal dominant polycystic kidney disease exosomes, 150 that distinguished between exosomes and microvesicles in the autosomal dominant polycystic kidney disease samples, and 62 that distinguished between exosomes and microvesicles in the medullary sponge kidney samples (Supplemental Table 1, Supplemental Figures 6 and 7). Support vector machine learning and partial least squares discriminant analysis were then used to highlight the proteins that maximize the discrimination between different sample types, revealing a core panel of 34 proteins that allowed us to distinguish the four conditions with an accuracy of 100% (Figure 4, A and B). After Z-score analysis, we built a heat map of the corresponding expression profiles (Figure 4A) and prepared a graphical representation for their cluster separation (Figure 4B).
Figure 3.
Volcano plots of univariate statistical analysis as applied to urinary extracellular vesicle samples. The plots are on the basis of the fold change (log2) and the P-value (−log10) of all proteins identified in (A) Mv from MSK and ADPKD; (B) Ex from MSK and ADPKD; (C) Mv and Ex from ADPKD; (D) Mv and Ex from MSK. Red circles indicate proteins related to the selected clinical trait with statistically significant changes between the clinical traits selected in this study. ADPKD, autosomal dominant polycystic kidney disease; MSK, medullary sponge kidney; Mv, microvesicles; Ex, exosomes.
Figure 4.
Proteins and gene ontology annotation that maximize the discrimination among all conditions. (A) Heat map of 34 core proteins identified through the combined use of univariate statistical analysis, support vector machine learning, and partial least squares discriminant analysis. In the heat map, each row represents a protein, and each column corresponds to a condition. Normalized Z scores of protein abundance are depicted by a pseudocolor scale, with red indicating positive expression, white indicating equal expression, and blue indicating negative expression compared with each protein value, whereas the dendrogram displays the outcome of unsupervised hierarchical clustering analysis, placing similar proteome profile values near to each other. (B) Two-dimensional scatter plot of multidimensional scaling analysis of exosome (solid symbols) and microvesicle (open symbols) of medullary sponge kidney (MSK; red triangles) and autosomal dominant polycystic kidney disease (ADPKD; black squares) samples using the above 34 highlighted proteins. Ellipses indicates 95% confidence intervals. Visual inspection of the dendrogram and heat map shows the ability of these proteins to clearly distinguish between the different conditions. (C) The heat map shows biologic process enrichment for different extracellular vesicle samples. In the heat map, each row represents a protein, and each column corresponds to a condition. Normalized Z scores of protein abundance are depicted by a pseudocolor scale, with red indicating positive expression, white indicating equal expression, and blue indicating negative expression compared with each protein value, whereas the dendrogram displays the outcome of unsupervised hierarchical clustering analysis, placing similar proteome profile values near to each other. Visual inspection of the dendrogram and heat map shows the ability of these biologic processes to distinguish between the different types of extracellular vesicle in the MSK and ADPKD samples. (D) ELISA for CD133. Box plot showing the median and interquartile range value of the urinary exosome CD133 in all samples. CD133 was highly expressed in patients with ADPKD compared with patients with MSK and healthy controls. (E) ROC curve analysis revealed that the expression of CD133 in urinary exosomes can discriminate patients with ADPKD from healthy controls and patients with MSK. AUC, area under the curve. ROC, Receiver Operating Characteristic; Mv, microvesicles; Ex, exosomes; CTR, healthy controls.
The diversity of expression profiles among the proteins in this core panel indicated their association with different functions, and therefore, GO analysis of functional annotations was used to build a scatter plot of enriched gene signatures on the y axis and –log10 P values on the x axis (Supplemental Figure 8). The size of scatters is proportional to the number of proteins associated with each biologic process. After Z-score analysis, we built a heat map showing the expression profiles of the enriched biochemical pathways (Figure 4C). Interestingly, this revealed that proteins involved in cell migration/adhesion were over-represented in the microvesicles of patients with polycystic kidney disease, whereas those involved in the regulation of the epithelial cell differentiation were over-represented in the exosomes of patients with autosomal dominant polycystic kidney disease.
ELISA for CD133 in Exosomes-Validated Proteomics
A homemade ELISA for urinary CD133 was performed in exosomes from all patients and healthy controls to validate proteomic data. We found that CD133 was highly expressed in patients with autosomal dominant polycystic kidney disease compared with patients with medullary sponge kidney and healthy controls (Figure 4D). The medians (interquartile ranges [IQRs]) were 1.04 (IQR, 0.54–1.68), 0.4 (IQR, 0.22–0.76), and 0.28 (IQR, 0.16–0.34) for patients with autosomal dominant polycystic kidney disease, patients with medullary sponge kidney, and healthy controls, respectively, and P values were P<0.001 for Kruskal–Wallis test analysis. Also, ROC analysis revealed that the expression of CD133 in urinary exosomes can discriminate patients with autosomal dominant polycystic kidney disease from healthy subjects and patients with medullary sponge kidney. The areas under the curve, the 95% confidence intervals (95% CIs), and P values of ROC analysis were 0.98 (95% CI, 0.94 to 1) and P<0.001 (patients with autosomal dominant polycystic kidney disease versus healthy controls), 0.82 (95% CI, 0.67 to 0.97) and P=0.003 (patients with autosomal dominant polycystic kidney disease versus patients with medullary sponge kidney), and 0.70 (95% CI, 0.51 to 0.89) and P=0.05 (patients with medullary sponge kidney versus healthy controls) (Figure 4E). The cutoff, sensitivity, specificity, and likelihood ratio are reported in Supplemental Table 2.
Discussion
Microvesicles and exosomes are known to be involved in the pathogenesis of several chronic kidney disorders, but few studies have focused on their role in kidney cystic diseases (9,11,25,26), and their potential involvement in medullary sponge kidney disease has not been addressed. In this study, we used mass spectrometry to identify the protein content of microvesicles and exosomes to gain insight into medullary sponge kidney–related cystogenesis and its similarities and differences compared with autosomal dominant polycystic kidney disease. By applying a layered statistical analysis approach, we found 34 core proteins that distinguished the microvesicles and exosomes of medullary sponge kidney and autosomal dominant polycystic kidney disease. Interestingly, most of these proteins were assigned to a small number of specific functions, including the regulation of epithelial cell differentiation, kidney development, cell migration, cell adhesion, carbohydrate metabolism, and extracellular matrix organization.
One of the core proteins was prominin 1 (CD133), a pentaspan transmembrane glycoprotein that localizes to membrane protrusions and is often expressed on adult stem/progenitor kidney cells, where it is thought to maintain stem cell properties by suppressing differentiation. The high-level expression of prominin 1 is associated with several types of cancer (27–29). This protein was more abundant in the exosomes of patients with autosomal dominant polycystic kidney disease, reflecting the attempted tissue repair in response to the aberrant rate of proliferation and apoptosis, which would require kidney progenitor cells. The upregulation of other proteins involved in cell migration/adhesion, such as Cadherin 4, or the epithelial cell differentiation, such as CREG1, seems to confirm this hypothesis. Accordingly, the kidney progenitor cells in human kidney papillary loops of Henle can differentiate into both neural-like and epithelial-like lineages as well as producing tubules (30). An abundant population of CD133+ cells was also shown to be present in the cystic wall and kidney tubules of patients with autosomal dominant polycystic kidney disease (31). The role of these cells is not yet clear, but it would be interesting to evaluate more patients with autosomal dominant polycystic kidney disease at different disease stages (from asymptomatic to the late disease stage) and clarify whether CD133+ (and CD24+) cells are associated with a better or worse prognosis.
We also found that the cellular repressor of E1A stimulated genes 1 (CREG1), a factor that interacts with the IGF2 receptor to regulate cell growth, was more abundant in autosomal dominant polycystic kidney disease. This protein may facilitate stem cell differentiation and activity, which was recently shown for the differentiation of embryonic stem cells in cardiomyocytes, improving the integration of stem cell–derived cardiomyocytes into recipient hearts (32). The exosomes sourced from our patients with autosomal dominant polycystic kidney disease not only contained higher levels of the proliferation regulator CREG1 but also, proteins required for matrix remodeling (ITIH5) and the regulation of salt secretion (GUCA2B or MAL). All of these mechanisms are important for cyst formation and enlargement, which in autosomal dominant polycystic kidney disease, involve tubular cell proliferation, abnormalities in the extracellular matrix, and transepithelial fluid secretion directed toward the cyst lumen. Because cysts are anatomically separated from their source tubule (33), the intracystic fluid does not originate from the glomerular filtrate, but rather, it originates from transepithelial fluid secretion (34).
Autosomal dominant polycystic kidney disease is also characterized by the disruption of the planar cell polarity pathway, which is required for oriented cell division and convergent extension to establish and maintain the structure of kidney tubules (35). We found that the FAT Atypical Cadherin 4 protein was more abundant in the exosomes of patients with autosomal dominant polycystic kidney disease. The loss of this protein disrupts oriented cell division and tubule elongation during kidney development, causing tubule dilation (36).
Notably, none of the proteins discussed above were upregulated in medullary sponge kidney, showing a different mechanism of cystogenesis. The specific diagnosis of medullary sponge kidney requires the anatomic feature of papillary precalyceal ectasias, sometimes associated with tiny medullary cysts, and such alterations can be unilateral or even limited to a portion of a single kidney medulla. Unlike autosomal dominant polycystic kidney disease, the tubular dilations and microcysts tend to be stable in terms of size throughout life as if they formed at the same time as the kidneys. Taken together, our data confirmed earlier reports indicating that medullary sponge kidney is an inborn malformation similar to developmental disorders, such as congenital hemihypertrophy and Beckwith–Wiedemann syndrome, and kidney developmental anomalies, such as horse-shoe kidney, unilateral kidney aplasia, and contralateral congenital small kidney (37,38), with the absence of the sequence of events leading to cyst formation. Additionally, our data showing the abundance of proteins involved in cell proliferation and extracellular matrix remodeling in patients with autosomal dominant polycystic kidney disease could in part explain why these patients are predisposed to the development of cancer, particularly kidney carcinoma (39).
In contrast, only a few proteins were highly expressed in medullary sponge kidney, mainly in the microvesicles. One example was SPP1 (osteopontin), a protein implicated in nephrolithiasis, a major clinical condition associated with medullary sponge kidney (13). Osteopontin is intimately involved in the regulation of both physiologic and pathologic mineralization. In normal bone tissue, osteopontin is expressed by osteoclasts and osteoblasts during bone remodeling, and osteoclast-derived osteopontin inhibits the formation of hydroxyapatite during normal mineralization (40). Osteopontin is also involved in kidney stone formation (41). This protein is synthesized in the kidney and secreted into the urine by epithelial cells, including the loop of Henle, distal convoluted tubule, and papillary epithelium (42), inhibiting the nucleation, growth, and aggregation of calcium oxalate crystals (43) and the binding of calcium oxalate crystals to kidney epithelial cells (44). Osteopontin knockout mice are hyperoxaluric, leading to the significant intratubular deposition of calcium oxalate, whereas wild-type mice remove calcium oxalate effectively (45). Therefore, the greater abundance of osteopontin in the microvesicles of our patients with medullary sponge kidney could represent a defense mechanism against microcalcification, and it could, at least partially, explain the bone symptoms often observed in patients with this disease. Accordingly, 58% of patients with medullary sponge kidney have a dual-energy x-ray absorptiometry profile of osteopenia, and 14% have a profile of osteoporosis unrelated to the common causes of bone demineralization, particularly hyperparathyroidism and menopause (46).
Taken together, our results have shown for the first time that the urinary microvesicles and exosomes of patients with autosomal dominant polycystic kidney disease and patients with medullary sponge kidney have distinct proteomic profiles. The urine of patients with autosomal dominant polycystic kidney disease was enriched for proteins involved in cell proliferation and matrix remodeling, probably due to pathologic tissue remodeling prompting cystic development and enlargement. In contrast, the urine of patients with medullary sponge kidney revealed a proteome indicative of a systemic biochemical imbalance that could explain the predisposition of such patients to parenchymal calcium deposition/nephrolithiasis and extrarenal complications, including bone mineralization defects.
Although small sample size and lack of independent replication are major weaknesses of the study and additional research is required for validation, some of the proteins (mainly CD133) that we identified could be suitable in the future as diagnostic biomarkers that could help clinicians to distinguish between patients with medullary sponge kidney and patients with autosomal dominant polycystic kidney disease during the early stages of the disease, avoiding time-consuming and expensive clinical testing.
Disclosures
Dr. Antonini, Dr. Antonucci, Dr. Bartolucci, Dr. Bruschi, Dr. Candiano, Dr. Del Zotto, Dr. Fabris, Dr. Gambaro, Dr. Granata, Dr. Ghiggeri, Dr. Lupo, Dr. Petretto, Dr. Santucci, and Dr. Zaza have nothing to disclose.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12191018/-/DCSupplemental.
Supplemental Material. Methods.
Supplemental Figure 1. Age and eGFR of all study participants included in the study.
Supplemental Figure 2. Characterization of isolated exosomes and microvesicles.
Supplemental Figure 3. Gene Ontology annotation of urinary extracellular vesicle proteins.
Supplemental Figure 4. Multidimensional scaling analysis of extracellular vesicles from the urine of patients with medullary sponge kidney (MSK) and patients with autosomal dominant polycystic kidney disease (ADPKD).
Supplemental Figure 5. Sample clustering and trait indicators.
Supplemental Figure 6. Venn diagram of statistically significant differences in protein abundance in the different types of extracellular vesicles from patients with medullary sponge kidney (MSK) or patients with autosomal dominant polycystic kidney disease (ADPKD).
Supplemental Figure 7. Proteins network interaction.
Supplemental Figure 8. Gene ontology enrichment analysis for core discriminatory proteins in the extracellular vesicles of patients with medullary sponge kidney (MSK) and patients with autosomal dominant polycystic kidney disease (ADPKD).
Supplemental Table 1. List of all significant proteins identified using mass spectrometry.
Supplemental Table 2. ELISA cutoff, sensitivity, specificity, and likelihood ratio.
Supplementary Material
Acknowledgments
This study was performed (in part) in the Laboratorio Universitario di Ricerca Medica Research Center, University of Verona.
This study was supported by Fondazione Cariverona call 2016 (Principal Investigator Prof. Tagliaro) and Ministero Della Salute grant GR-2011-02350438.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ: Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94: 3791–3799, 1999 [PubMed] [Google Scholar]
- 2.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ: Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 20: 1487–1495, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Dear JW, Street JM, Bailey MA: Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 13: 1572–1580, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014 [DOI] [PubMed] [Google Scholar]
- 5.van Balkom BW, Pisitkun T, Verhaar MC, Knepper MA: Exosomes and the kidney: Prospects for diagnosis and therapy of renal diseases. Kidney Int 80: 1138–1145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mause SF, Weber C: Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO: Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Thongboonkerd V, McLeish KR, Arthur JM, Klein JB: Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int 62: 1461–1469, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon PG, You S, Lee JE, Hwang D, Baek MC: Urinary exosomes and proteomics. Mass Spectrom Rev 30: 1185–1202, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ: Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20: 278–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salih M, Demmers JA, Bezstarosti K, Leonhard WN, Losekoot M, van Kooten C, Gansevoort RT, Peters DJ, Zietse R, Hoorn EJ; DIPAK Consortium : Proteomics of urinary vesicles links plakins and complement to polycystic kidney disease. J Am Soc Nephrol 27: 3079–3092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambaro G, Danza FM, Fabris A: Medullary sponge kidney. Curr Opin Nephrol Hypertens 22: 421–426, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Fabris A, Lupo A, Ferraro PM, Anglani F, Pei Y, Danza FM, Gambaro G: Familial clustering of medullary sponge kidney is autosomal dominant with reduced penetrance and variable expressivity. Kidney Int 83: 272–277, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Torregrossa R, Anglani F, Fabris A, Gozzini A, Tanini A, Del Prete D, Cristofaro R, Artifoni L, Abaterusso C, Marchionna N, Lupo A, D’Angelo A, Gambaro G: Identification of GDNF gene sequence variations in patients with medullary sponge kidney disease. Clin J Am Soc Nephrol 5: 1205–1210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabris A, Bruschi M, Santucci L, Candiano G, Granata S, Dalla Gassa A, Antonucci N, Petretto A, Ghiggeri GM, Gambaro G, Lupo A, Zaza G: Proteomic-based research strategy identified laminin subunit alpha 2 as a potential urinary-specific biomarker for the medullary sponge kidney disease. Kidney Int 91: 459–468, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Ria P, Fabris A, Dalla Gassa A, Zaza G, Lupo A, Gambaro G: New non-renal congenital disorders associated with medullary sponge kidney (MSK) support the pathogenic role of GDNF and point to the diagnosis of MSK in recurrent stone formers. Urolithiasis 45: 359–362, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Fabris A, Ferraro PM, Comellato G, Caletti C, Fantin F, Zaza G, Zamboni M, Lupo A, Gambaro G: The relationship between calcium kidney stones, arterial stiffness and bone density: Unraveling the stone-bone-vessel liaison. J Nephrol 28: 549–555, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Muto S, Ohmoto Y, Iwata F, Fujiki H, Mori T, Yan L, Horie S: Exploring urinary biomarkers in autosomal dominant polycystic kidney disease. Clin Exp Nephrol 19: 968–973, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mäger I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R: Methodological guidelines to study extracellular vesicles. Circ Res 120: 1632–1648, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Kulak NA, Pichler G, Paron I, Nagaraj N, Mann M: Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat Methods 11: 319–324, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Chawade A, Alexandersson E, Levander F: Normalyzer: A tool for rapid evaluation of normalization methods for omics data sets. J Proteome Res 13: 3114–3120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langfelder P, Horvath S: WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics 9: 559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, Wang NS, Knepper MA: Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan MC, Bakeberg JL, Gainullin VG, Irazabal MV, Harmon AJ, Lieske JC, Charlesworth MC, Johnson KL, Madden BJ, Zenka RM, McCormick DJ, Sundsbak JL, Heyer CM, Torres VE, Harris PC, Ward CJ: Identification of biomarkers for PKD1 using urinary exosomes. J Am Soc Nephrol 26: 1661–1670, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigmann A, Corbeil D, Hellwig A, Huttner WB: Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A 94: 12425–12430, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbeil D, Röper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB: The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem 275: 5512–5520, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D: Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res 319: 15–26, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ward HH, Romero E, Welford A, Pickett G, Bacallao R, Gattone VH 2nd, Ness SA, Wandinger-Ness A, Roitbak T: Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta 1812: 1344–1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodi D, Ligabue G, Cavazzini F, Lupo V, Cappelli G, Magistroni R: CD133 and CD24 expression in renal tissue of patients affected by autosomal dominant polcystic kidney disease. Stem Cell Discovery 3: 211–217, 2013 [Google Scholar]
- 32.Liu J, Qi Y, Li S, Hsu SC, Saadat S, Hsu J, Rahimi SA, Lee LY, Yan C, Tian X, Han Y: CREG1 interacts with Sec8 to promote cardiomyogenic differentiation and cell-cell adhesion. Stem Cells 34: 2648–2660, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Grantham JJ, Geiser JL, Evan AP: Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int 31: 1145–1152, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Terryn S, Ho A, Beauwens R, Devuyst O: Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1812: 1314–1321, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J: Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol 21: 1521–1532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Lambrianides AL, John DR: Medullary sponge disease in horseshoe kidney. Urology 29: 426–427, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Gambaro G, Fabris A, Citron L, Tosetto E, Anglani F, Bellan F, Conte M, Bonfante L, Lupo A, D’Angelo A: An unusual association of contralateral congenital small kidney, reduced renal function and hyperparathyroidism in sponge kidney patients: On the track of the molecular basis. Nephrol Dial Transplant 20: 1042–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Yu TM, Chuang YW, Yu MC, Chen CH, Yang CK, Huang ST, Lin CL, Shu KH, Kao CH: Risk of cancer in patients with polycystic kidney disease: A propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol 17: 1419–1425, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Hunter GK, Kyle CL, Goldberg HA: Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J 300: 723–728, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinman JG, Wesson JA, Hughes J: Osteopontin and calcium stone formation. Nephron, Physiol 98: 43–47, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Giachelli CM, Pichler R, Lombardi D, Denhardt DT, Alpers CE, Schwartz SM, Johnson RJ: Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int 45: 515–524, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Worcester EM, Beshensky AM: Osteopontin inhibits nucleation of calcium oxalate crystals. Ann N Y Acad Sci 760: 375–377, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Wesson JA, Worcester E: Formation of hydrated calcium oxalates in the presence of poly-L-aspartic acid. Scanning Microsc 10: 415–424, 1996 [PubMed] [Google Scholar]
- 45.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J: Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14: 139–147, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Fabris A, Bernich P, Abaterusso C, Marchionna N, Canciani C, Nouvenne A, Zamboni M, Lupo A, Gambaro G: Bone disease in medullary sponge kidney and effect of potassium citrate treatment. Clin J Am Soc Nephrol 4: 1974–1979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.