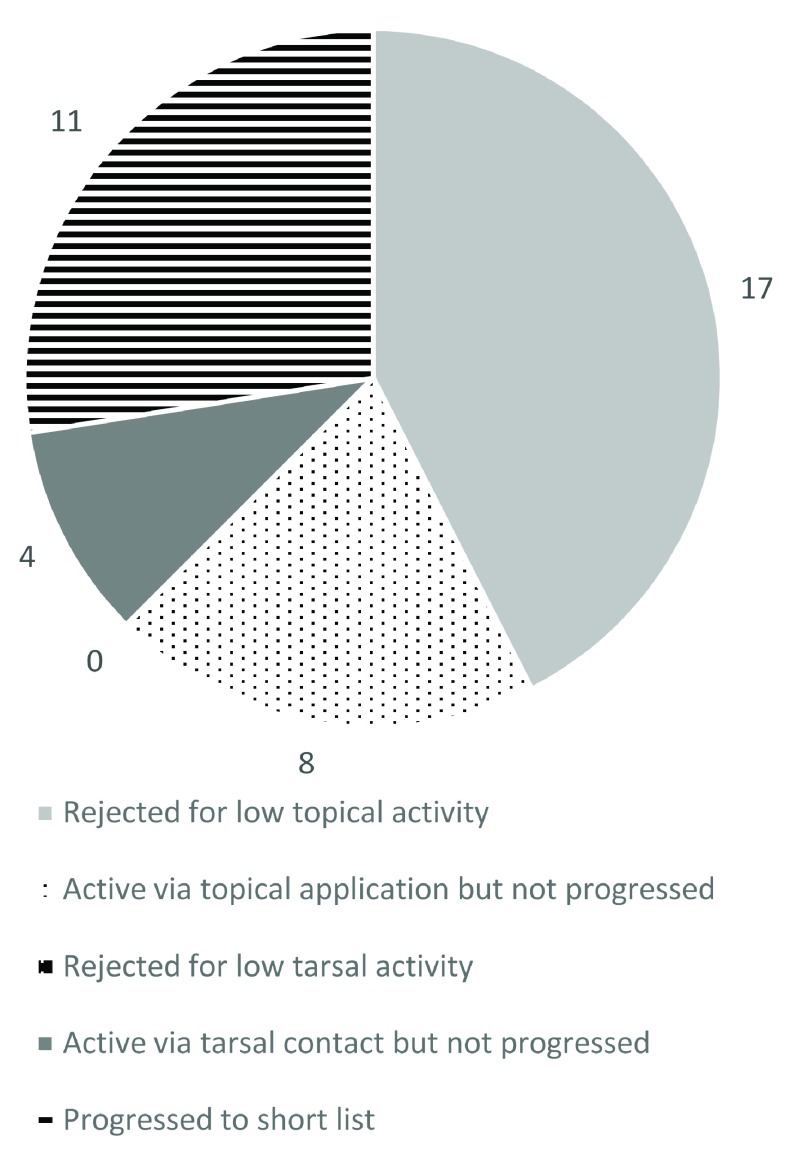
Figure 2. Fate of the 40 compounds taken through the testing cascade.
In general, compounds which were active (≥80% mortality 24 hours after exposure) in topical application were progressed to tarsal contact assays, and those active at this step were progressed to measure relative potency through determining their discriminating dose. No compounds were therefore rejected for low tarsal activity. However, of the 23 compounds which were active when applied topically, not all were progressed to tarsal contact testing due to a range of considerations including physicochemical properties and regulatory challenges foreseen in their development. Of the compounds which were active in tarsal contact assays, only a representative compound from each IRAC MoA class were progressed, with the exception of fenpyroximate and tolfenpyrad, both from class 21A.