Abstract
Introduction
Medicare claims data may be a rich data source for tracking population dementia rates. Insufficient understanding of completeness of diagnosis, and for whom, limits their use.
Methods
We analyzed agreement in prevalent and incident dementia based on cognitive assessment from the Health and Retirement Study for persons with linked Medicare claims from 2000 to 2008 (N = 10,450 persons). Multinomial logistic regression identified sociodemographic factors associated with disagreement.
Results
Survey-based cognitive tests and claims-based dementia diagnosis yielded equal prevalence estimates, yet only half were identified by both measures. Race and education were associated with disagreement. Eighty-five percent of respondents with incident dementia measured by cognitive decline received a diagnosis or died within the study period, with lower odds among blacks and Hispanics than among whites.
Discussions
Claims data are valuable for tracking dementia in the US population and improve over time. Delayed diagnosis may underestimate rates within black and Hispanic populations.
Keywords: Prevalence, Incidence, Diagnosis, Cognition, Race/ethnicity, Disparities
1. Introduction
Accurate estimates of the prevalence and incidence of dementia, how they are changing over time, and for whom are essential for quantifying disease burden and for preparing health and long-term care systems for the inevitable increase in cases. Yet, there is no single data source for doing so. In the absence of dementia tracking through a national screening program, the main sources for estimating dementia in the US are nationally representative surveys and health-care claims.
Medicare claims are an important data source for identifying and tracking rates of diagnosed disease over time in the older US population because the program provides health insurance for about 97% of older Americans from the age of 65 years until death. The number of diagnosed cases in the Medicare records, however, may underestimate the actual burden of disease if individuals do not seek treatment for symptoms or request cognitive assessments. Providers do not recognize symptoms and/or undertake assessment or choose not to report it because of a lack of treatments that can change the course of the disease [1], [2], [3]. Nationally representative surveys are another key source for estimating population dementia prevalence. The Health and Retirement Study (HRS) [4], [5], [6] and the National Health and Aging Trends Study have repeatedly used cognitive tests to measure dementia prevalence as well as onset in nationally representative cohorts. Cognitive tests for dementia ascertainment from surveys have been criticized for focusing heavily on language and memory [7], being sensitive to education level [8], and for their limited ability to differentiate mild cognitive impairment from dementia [9].
Prior validation studies were limited by small or nonrepresentative samples. Taylor et al. [10] compared dementia diagnoses in Medicare claims to clinical examinations in the 2001–2003 Aging Demographic and Memory Study (ADAMS), a small subsample of the HRS with few minority respondents, and reported sensitivity of 85% and a specificity of 89%. Other validation studies comparing claims and clinical assessment were based on nonrepresentative samples and similarly reported coexistence of false-positive and negative diagnoses in claims data [11], [12], [13], [14], [15].
Studies that compared claims-based diagnoses with survey-based cognitive assessments for dementia ascertainment in samples broadly representative of the older US population had opposite findings. Two studies reported higher dementia ascertainment in Medicare claims data than survey-based ascertainment [16], [17]. In contrast, Amjad et al. [18] reported that 60% of respondents with “probable” dementia in 2011 National Health and Aging Trends Study data had formal diagnosis in three-year Medicare claims. None of these studies addressed the measurement error in dementia ascertainment based on survey data that two recent studies showed, and this led to an upward bias in dementia ascertainment [6], [19]. They did not address the measurement error in claims data due to the “rule-out” diagnosis of reversible dementia symptoms (e.g., visual or auditory problems, vitamin B12 deficiency, thyroid disturbance). Although study results were inconsistent, they regularly found that the level of agreement in dementia prevalence across data sources varied with the characteristics of the individual [10], [12], [13], [15], [17], [18], [20].
In this study, we analyzed dementia prevalence and incidence in a large sample of individuals broadly representative of the older US population from the HRS with data linkages to their Medicare claims records from 2000 to 2008. We improved upon the methods used in prior studies by requiring verification of dementia in both survey and claims-based data sources to reduce measurement error. We added to prior literature an analysis of how (dis)agreement in dementia prevalence is changing over time and for which populations. This is the first study to quantify concordance in incidence of dementia and the timing of diagnosis after substantial cognitive decline, as well as racial/ethnic, socioeconomic, and sex differences in this timing. The findings illuminate the value and caveats of using Medicare claims and cognitive measures from survey data for studying dementia in the US population. This is particularly important given the absence of clinical assessments in nationally representative, large, and longitudinal samples. Diagnoses in Medicare claims reflect clinical practice, and an improved understanding of who is being diagnosed and when may aid policies to reduce disparities in dementia diagnosis.
2. Methods
2.1. Study population
We use data from the HRS linked to respondents' Medicare claims from the beginning of 2000 to the end of 2008. HRS is a nationally representative longitudinal study that has surveyed Americans older than 50 years and their spouses since 1992. Respondents are interviewed biennially, on topics of health, health-care usage, employment, economy, and family. A key feature of the HRS study design is oversampling of African Americans and Hispanics, and weights may be used for providing a nationally representative sample. Minority response rates at baseline and in longitudinal follow-ups have been equal to or better than those of majority whites [21]. Eighty-eight percent of HRS respondents consented to the linkage of their survey responses to their Medicare claims records [22]. Our sample is restricted to HRS respondents aged 67 years and older, with linked claims data and at least two years of continuous fee-for-service (FFS) enrollment yielding 10,450 unique persons and 31,186 person-waves. The mean follow-up was 2.98 HRS interview waves.
2.2. Dementia measures and outcomes
Cognitive tests were administered at each wave to respondents using an adapted version of the Telephone Interview for Cognitive Status. When missing for self-respondents, the measures were imputed by HRS as described by Fisher et al. [23]. Around 6.2% of self-respondents in our study sample had at least one imputed score for cognitive tests. When a respondent does no, or cannot perform the cognitive assessment, dementia was determined using information provided by a proxy respondent, typically a spouse or other family member and the interviewer [21]. We followed prior studies on the classification of dementia which is based on the concordance of HRS cognitive functioning scores and consensus diagnosis of dementia in a subset of HRS respondents who had extensive neuropsychological assessment in ADAMS [4], [24]. An individual was classified as having dementia based on a low score (0-6 out of 27) on test items that evaluate memory and concentration and executive function: immediate and delayed word recall, counting back from 100 by 7's, and counting back from 20 [4], [5], [24]. Among respondents with a proxy, dementia is based on a number of limitations with instrumental activities of daily living, interviewer impairment rating from 0 (none) to 2 (cognitive limitations), and proxy informants' impairment rating from 0 (none) to 4 (poor).
To reduce measurement error in dementia ascertainment based on cognitive scores, we required one wave with dementia and evidence of continued cognitive impairment in the next consecutive wave [6], [19]. If the respondent with one wave of dementia died before the next wave, he or she was assumed to have dementia before dying. Once we identified a respondent as having “verified” dementia, we assumed dementia in all subsequent waves.
Providers that bill Medicare use codes for patient diagnoses. The first code listed is the primary diagnosis, and more than one diagnosis code is allowed. In Medicare claims, we ascertained dementia based on the Chronic Conditions Data Warehouse algorithm for Alzheimer's disease or related disorders or senile dementia using the following International Classification of Disease, ninth revision diagnosis codes: 331.0, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.10, 294.11, 294.20, 294.21, 294.8, and 797. Additional diagnostic codes were also included to account for dementia with Lewy bodies, cerebral degeneration, senile psychosis, and dementia classified elsewhere: 331.82, 331.89, 331.9, 290.8, 290.9, 294.9. Chronic Conditions Data Warehouse algorithm requires at least one inpatient, facility, home health, or outpatient claim with one of the aforementioned diagnosis codes during a three-year look-back period. Similar to the verified measure in HRS, we additionally required a second diagnosis claim over the study period to rule out reversible dementia symptoms.
The main outcome of interest is the (dis)agreement between the two measures of dementia for an individual. Agreement at a point in time (prevalent dementia) was defined as having the same dementia status across the data sources during the years between two consecutive HRS waves, approximately two years. We assessed agreement in incident dementia similarly by comparing dates of incidence of dementia based on the two measures. Incident dementia using claims data is the earliest dementia diagnosis date on a claim conditional on no prior diagnosis (verified by subsequent diagnosis as described previously). Incident dementia identified using HRS is the earliest survey date of dementia based on cognitive assessment conditional on not having dementia as measured by scores in the prior waves. Again, verification of “new” dementia with a subsequent low cognitive status in the next wave is required.
2.3. Explanatory variables
Also included in the analysis are age, gender, race (black, Hispanic, non-Hispanic white), highest level of education (less than high school, high school, college and above), marital status (married or not), the presence of chronic conditions and diseases (stroke, heart disease, diabetes, and hypertension), health-care utilization (binary indicator for a physician visit during the past two years), and survival (indicator for whether died between survey waves).
2.4. Statistical analysis
We applied HRS sampling weights to quantify concordance in dementia prevalence from 2000 to 2008. We used multinomial logistic regression to quantify demographic and socioeconomic factors associated with concordance in dementia prevalence, adjusting for survival into the next wave, physician visits, and a linear time trend. An interaction term between race and time was tested separately to see whether there were differential time trends by race.
We quantified dementia incidence in a sample without prior dementia based on either measure. We also selected a subsample with incident dementia between 2000 and 2004 based on cognitive tests from the HRS (N = 1161) and analyzed whether and when a dementia diagnosis occurred based on claims data. We quantified timing and applied sampling weights. We used multinomial logistic regression to quantify the socioeconomic and demographic factors associated with the timing of diagnosis relative to incident dementia based on cognitive decline from “no dementia” to “new” dementia based on scores from cognitive tests.
As a sensitivity check, we modified the definition of dementia in the following ways: (1) required any subsequent verification of dementia in HRS (rather than that at the next consecutive wave); (2) required no verification for diagnosis in claims; and (3) used an augmented list of diagnostic codes including dementia symptoms (International Classification of Disease, ninth revision codes: 780.93, 784.3, 784.69, and 331.83). When defining agreement, we allowed for a longer period for diagnosis or HRS dementia (extending by approximately 2 years). We also added control for household wealth in multivariate analyses.
3. Results
3.1. Sample characteristics
Table 1 reports the cross-sectional characteristics of the respondents in years 2000 and 2008. Characteristics in this linked sample were compared with those in the full HRS sample aged 67 years and older. In 2000, the linked sample was comparable to the full HRS sample in terms of gender, education, marital status, and cardiovascular profiles. The linked sample is more likely to be non-Hispanic white than the full HRS sample.
Table 1.
Sample characteristics in years 2000 and 2008
| Characteristics | HRS claims linked sample |
HRS 67 + sample |
P values |
|||
|---|---|---|---|---|---|---|
| 2000 | 2008 | 2000 | 2008 | 2000 | 2008 | |
| N | 6142 | 5706 | 9404 | 10,285 | ||
| Age, % | .169 | .000 | ||||
| 67–74 | 42.6 | 39.3 | 46.7 | 45.0 | ||
| 75–84 | 42.8 | 41.1 | 40.4 | 38.4 | ||
| 85 and above | 14.6 | 19.6 | 12.9 | 16.6 | ||
| Mean (SD), years | 76.8 (6.80) | 77.6 (7.17) | 76.2 (6.78) | 76.6 (7.16) | .049 | .000 |
| Female, % | 59.8 | 60.0 | 59.2 | 58.1 | .369 | .149 |
| Race, % | .010 | .000 | ||||
| White | 86.8 | 87.5 | 86.4 | 84.6 | ||
| Black | 9.0 | 8.0 | 8.5 | 8.4 | ||
| Hispanic | 4.1 | 4.5 | 5.1 | 7.0 | ||
| Education, % | .893 | .761 | ||||
| Less than high school | 35.5 | 25.9 | 35.3 | 28.3 | ||
| High school and equivalent | 32.1 | 34.1 | 31.5 | 33.2 | ||
| College and above | 32.4 | 40.0 | 33.2 | 38.4 | ||
| Not married/partnered, % | 47.5 | 47.2 | 46.9 | 45.1 | .474 | .041 |
| Cardiovascular risk factors, % | ||||||
| Stroke | 12.8 | 13.4 | 12.1 | 12.8 | .653 | .041 |
| Heart disease | 32.0 | 34.7 | 30.3 | 32.7 | .222 | .001 |
| Diabetes | 15.0 | 21.5 | 15.4 | 22.1 | .493 | .919 |
| Hypertension | 52.8 | 64.8 | 52.0 | 64.6 | .672 | .279 |
| Died between this and next wave, % | 12.2 | 11.3 | 11.9 | 12.4 | .021 | .028 |
NOTE: HRS 67 + sample requires (1) age ≥ 67 years and (2) responded to HRS interview. HRS claims–linked sample additionally requires continuous FFS enrollment for at least 2 years. The reported percentages are weighted using wave-specific HRS sampling weights to adjust for survey design. P values indicate the level of statistical difference in characteristics between HRS 67 + sample and HRS claims–linked sample.
Abbreviations: HRS, Health and Retirement Study; FFS, fee-for-service; SD, standard deviation.
3.2. Concordance in prevalent dementia
We reported concordance in prevalent dementia for persons according to four categories: (1) person does not have dementia, both measures; (2) has dementia, both measures; (3) has dementia based on cognitive tests only; and (4) has dementia based on diagnosis only, during years between two consecutive HRS survey waves. The first two categories were considered as agreement. There was concordance in prevalent dementia for 86.1% of the respondents based on two measures (Table 2). Dementia prevalence ascertained by both measures was 7.2% while that ascertained by survey-based cognitive tests only was 6.9% and by diagnosis only was 7.0%. Thus, only half of dementia cases identified by one source had dementia ascertained by the other measure. Whites had higher concordance (both has dementia or both does not) than blacks and Hispanics (W = 88.1 percent; B = 74.9 percent; H = 70.8 percent). “Has dementia both measures” was more prevalent in racial/ethnic minorities than whites (W = 6.7 percent; B = 12.2 percent; H = 9.4 percent). The dominant disagreement type among whites was “dementia by diagnosis only,” while that among blacks and Hispanics was “dementia by cognitive tests only.” Whites and Hispanics had a similar proportion of dementia by diagnosis only (W = 7.2%; H = 8.1%), nearly twice as high as that for blacks (B = 4.3%).
Table 2.
Concordance in dementia prevalence and by race 2000–2008 (N = 31,186)
| Concordance category | All | Whites | Blacks | Hispanics |
|---|---|---|---|---|
| No dementia, both measures, % | 78.9 | 81.4 | 62.7 | 61.4 |
| Dementia, both measures, % | 7.2 | 6.7 | 12.2 | 9.4 |
| Dementia, cognitive test only, % | 6.9 | 4.8 | 20.8 | 21.1 |
| Dementia, diagnosis only, % | 7.0 | 7.2 | 4.3 | 8.1 |
| Concordance in prevalent dementia, % | 86.1 | 88.1 | 74.9 | 70.8 |
| N | 31,186 | 25,504 | 3953 | 1728 |
NOTE: Agreement is based on the same dementia status during the years between two consecutive Health and Retirement Study waves.
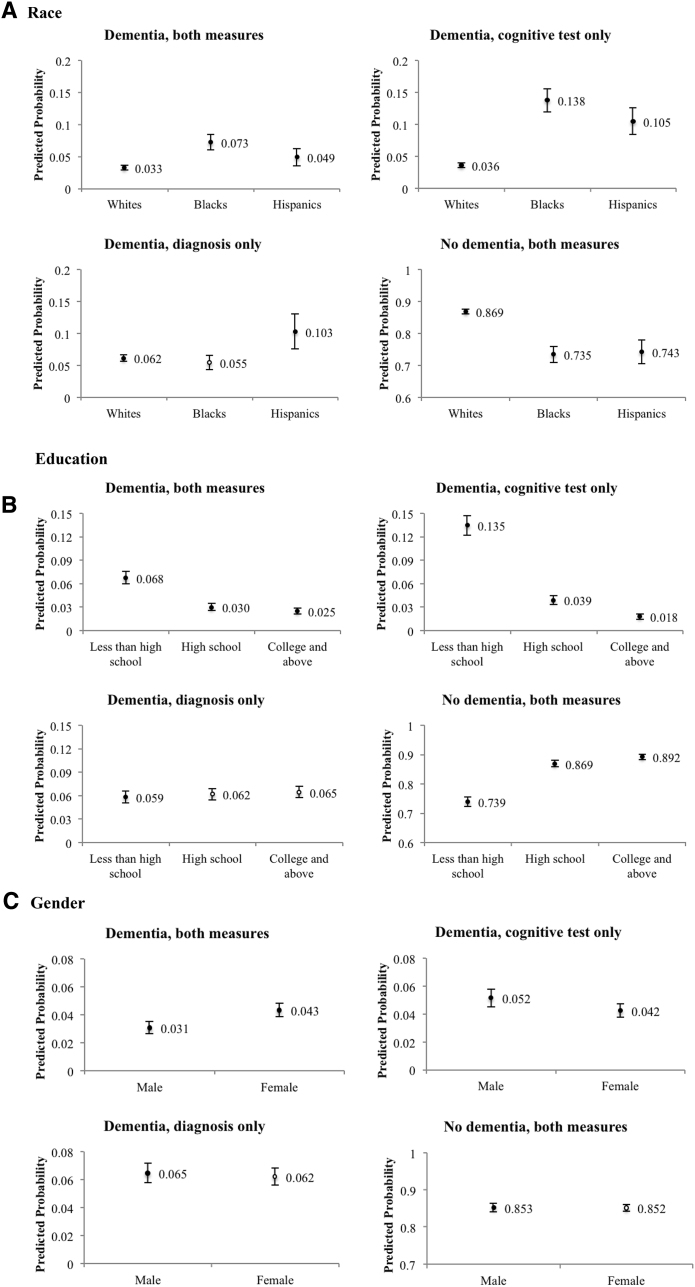
Fig. 1 shows the results from multinomial logits models of concordance across dementia measures, illustrated by predicted probabilities of each outcome separately by race/ethnicity, education, and sex. Odds ratios are provided in Supplementary Table 1. Blacks were 3.8 times as likely and Hispanics 2.9 times as likely as whites to have dementia identified by a cognitive test only (B = 0.138; W = 0.036; H = 0.105). The likelihood of having “dementia by diagnosis only” was 0.062 for whites and 0.055 for blacks, both not statistically different (P value = .268). The probability of “dementia by diagnosis only” for Hispanics was 0.103, which is statistically different than whites (P value = .004). We found no differential time trends by race.
Fig. 1.
Predicted probability of concordance in prevalent dementia by race (A), education (B), and gender (C) (N = 31,186). Predicted probabilities of each concordance category are based on estimates from multinomial logistic regression, adjusting for sex, age group, race, education, marital status, survival in two years, doctor visit during the past two years, and a linear time trend; error bars show 95% confidence intervals of predictions. Black dots indicate statistical difference between a probability and that for whites, less than high school education, or male, at a significance level of 0.05. The number of observations in regression reduced from 31,186 to 31,117 due to missing values in covariates.
Individuals with less than high school education (0.135) were more likely to have dementia ascertained by cognitive tests only than individuals with a high school (0.039) or college (0.018) education. There were no differences by education in the predicted probability of “dementia, diagnosis only.” Males were slightly more likely to have “dementia by cognitive tests only” (P value = .014), and there was no gender difference in the likelihood of “dementia by diagnosis only” (P value = .52). Over time, respondents were less likely to have “dementia by cognitive tests only” (Supplementary Table 2). All results were robust to varying definitions of dementia and of agreement, and to adding wealth controls to the models.
3.3. Concordance in incident dementia
Table 3 reported concordance in dementia incidence, during a 4-year time window (i.e., 2 years backward and 2 years forward). Roughly 83% of individuals had agreement in dementia incidence across the measures. Concordance among racial/ethnic minorities was lower than that among whites (W = 84.5 percent; B = 73.8 percent; H = 72.9 percent). Among whites, dementia incidence based on cognitive tests only (3.2%) was lower than that based on diagnosis only (12.3%). In contrast, among blacks, dementia incidence based on cognitive tests only (15.2%) was higher than that based on diagnosis only (11.1%). Among Hispanics, the rates were similar for cognitive tests only (13.2%) and diagnosis only (13.9%).
Table 3.
Concordance in dementia incidence and by race 2000–2008 (N = 9623)
| Concordance category | All | Whites | Blacks | Hispanics |
|---|---|---|---|---|
| No incident dementia, both measure, % | 79.1 | 81.4 | 68.2 | 69.3 |
| Incident dementia, both measure, % | 3.4 | 3.1 | 5.6 | 3.5 |
| Incident dementia, cognitive test only, % | 5.2 | 3.2 | 15.2 | 13.2 |
| Incident dementia, diagnosis only, % | 12.2 | 12.3 | 11.1 | 13.9 |
| Concordance in incident dementia, % | 82.5 | 84.5 | 73.8 | 72.9 |
| N | 9623 | 7883 | 1201 | 538 |
NOTE: Agreement is defined as having incident dementia by both measures, during a 4-year time window (i.e., 2 years backward and 2 years forward). Dementia based on cognitive test requires evidence of continued cognitive impairment in the next consecutive Health and Retirement Study (HRS) wave or death before the next wave. Dementia diagnosis ascertained by Medicare claims requires observing a second diagnosis or death as of December 31, 2008, as verification. This sample excludes respondents with incident dementia based on HRS before wave 5 (i.e., year 2000) given time horizon of this study and excludes those with incident dementia based on HRS at wave 9 (i.e., year 2008) due to availability of linked claims data up to December 31, 2008.
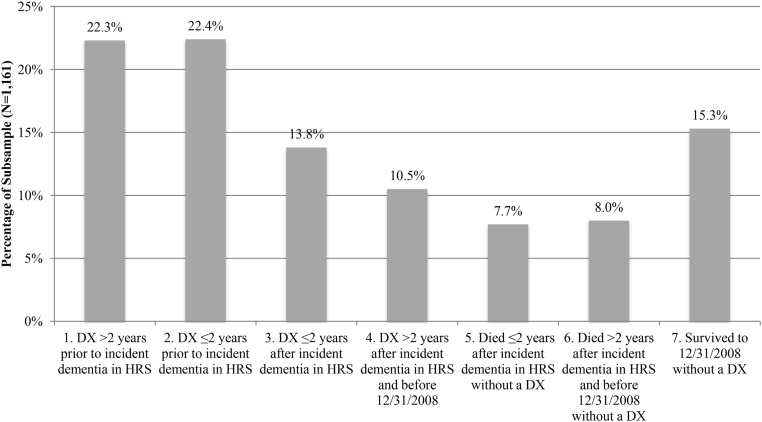
We analyzed diagnosis among a subsample of respondents with incident dementia ascertained with cognitive tests between HRS survey years 2000 and 2004. We quantified groups of individuals who were (1) diagnosed more than 2 years before incident dementia; (2) diagnosed ≤2 years before incident dementia; (3) diagnosed 2 years or sooner after incident dementia; (4) diagnosed more than 2 years after incident dementia and before the end of the study period (December 31, 2008); (5) died 2 years or sooner after incident dementia without a diagnosis; (6) died more than 2 years after incident dementia and before the end of the study period (December 31, 2008), without a diagnosis; and (7) survived to the end of the study period (December 31, 2008), without a diagnosis. A significant proportion (22.3%) of patients were diagnosed before incident dementia as measured by cognitive tests. About 85% were either diagnosed with dementia or died during the study period (Fig. 2). The remaining 15.3% of the sample were on average followed up for 5.9 years without receiving a diagnosis.
Fig. 2.
Timing of diagnosis in claims data relative to incident dementia based on cognitive scores in Health and Retirement Study data (N = 1161). DX = diagnosis coded in Medicare claims. This subsample is limited to respondents who were ascertained as dementia by HRS cognitive tests for the first time during HRS 2000, 2002, or 2004 waves. From the left to the right, outcomes are: (1) diagnosed more than 2 years before incident dementia in HRS, (2) diagnosed 2 years or less before incident dementia in HRS, (3) diagnosed 2 years or sooner after incident dementia in HRS, (4) diagnosed more than 2 years after incident dementia in HRS and before December 31, 2008, (5) died 2 years or sooner after incident dementia in HRS without a diagnosis in claims, (6) died more than 2 years after incident dementia in HRS and before December 31, 2008, without a diagnosis in claims, and (7) survived to December 31, 2008, without a diagnosis in claims. The reported percentages are weighted using wave-specific HRS sampling weights to adjust for survey design.
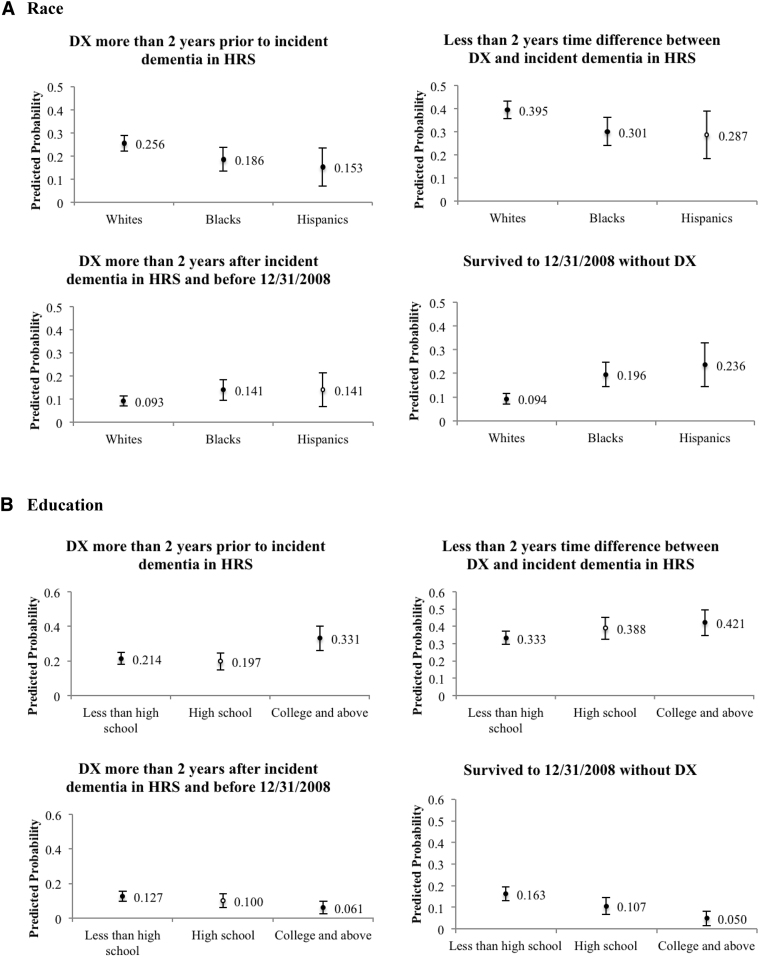
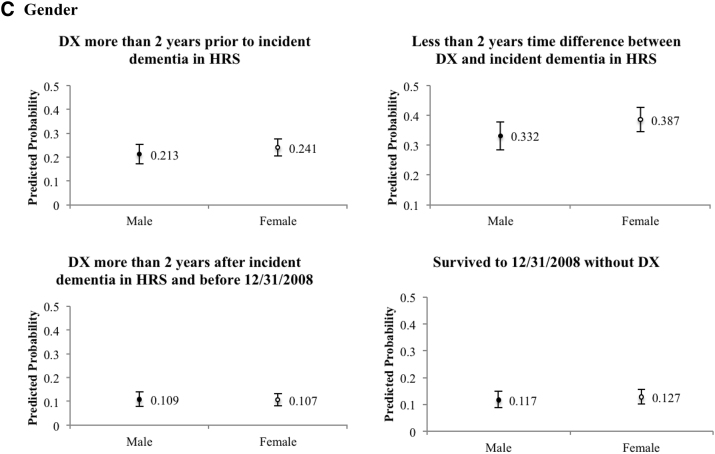
Descriptive characteristics of individuals in each of the 7 groups described previously are provided in Supplementary Tables 3 and 4. Fig. 3 illustrates with predicted probabilities the results of a multinomial logit model of the factors associated with timing of diagnosis relative to incident dementia as ascertained through cognitive tests. Odds ratios are available in Supplementary Table 5. The estimated model combined the groups (2) and (3), where the time difference between incident dementia and diagnosis is two years or less, and also combined the groups (5) and (6)—persons who died without a diagnosis and adjusted for sex, age group, race, education, marital status, doctor visit during the past two years, and a linear time trend. Blacks (0.196) and Hispanics (0.236) had a significantly higher odds of surviving without diagnosis than whites (0.094), and the differences were statistical significant (P valueB < .000; P valueH = .003). Blacks and Hispanics were less likely than whites to be diagnosed over 2 years before the incident dementia based on cognitive tests (P valueB = .027; P valueH = .022). Blacks (0.301) were less likely be diagnosed within 2 years of incident dementia than whites (0.395), and there were no statistical differences between Hispanics and whites (P value = .055). Blacks were more likely (0.141) than whites (0.093) to receive diagnosis 2 years after cognitive test–based dementia incidence (P value = .048); no significant difference was detected between Hispanics and whites (P value = .205).
Fig. 3.
Predicted probability of relative timing of incident dementia, by race (A), education (B), and gender (C) (N = 1161). DX = diagnosis coded in Medicare claims. Five outcome groups in the multinomial logistic regression include (1) diagnosed more than 2 years before incident dementia in HRS, (2) less than 2 years time difference between incident dementia in claims data and in HRS, (3) diagnosed more than 2 years after incident dementia in HRS and before December 31, 2008, (4) died before December 31, 2008, without a diagnosis in claims, and (5) survived to December 31, 2008, without a diagnosis in claims. This figure omits estimates for group (4). Predicted probabilities of each concordance category are based on estimates from multinomial logistic regression, adjusting for sex, age group, race, education, marital status, doctor visit during the past two years, and linear time trend; error bars show 95% confidence intervals of predictions. Black dots indicate statistical difference between a probability and that for whites, less than high school education, or male, at a significance level of 0.05. The number of observations in regression reduced from 1161 to 1152 due to missing values in covariates.
Disparities were prominent between those with less than high school education and those with any college education (Fig. 3). Individuals with a college education were more likely to have a diagnosis before dementia was first detected by cognitive tests (P value = .036) and less likely to have received a diagnosis more than two years after (P value = .005) and of surviving without a diagnosis (P value < .000). Results were not statistically different for those with a high school diploma compared with no high school degree, with the exception of a lower likelihood of surviving without a diagnosis.
Gender was not associated with existence and timing of diagnosis (Fig. 3). There was a decrease in the likelihood of diagnosis more than 2 years after the incident dementia, as well as an increase in that of surviving without a diagnosis (Supplementary Table 6). These results were not qualitatively different when the definition of dementia was modified and with wealth adjustments.
4. Discussion
Using a nationally representative sample of older Americans from the longitudinal HRS with linkages to their health-care claims, we found that at a point in time, ascertained dementia from survey-based cognitive tests and dementia diagnosis from Medicare claims produced similar prevalence estimates at the population level (14%). However, only half of these individuals were identified as having dementia by both measures. This level of agreement in dementia prevalence at the individual level was consistent with previous literature [16], [17], [18]. Racial/ethnic minorities, individuals with less than high school education, and males were more likely than whites, college-educated individuals, and females (respectively) to have been identified as with dementia based on cognitive tests only. In contrast, dementia ascertained by diagnosis only was no different across education groups, for males relative to females and blacks relative to whites. Hispanics were more likely than whites to have diagnosed with dementia only (but not ascertained by cognitive tests).
To our knowledge, this is the first study to examine concordance in the timing of incident dementia. We found almost one-quarter had a diagnosis of dementia two years or more before dementia was indicated by cognitive tests. This was more prevalent among college-educated person than those with lower levels of education. Among respondents with incident dementia between the years 2000 and 2004 (ascertained with cognitive assessment), 85% of these respondents were diagnosed or had died by 2008. Although only 15% had not yet been diagnosed, it was more common among blacks and Hispanics than whites. Unmeasured factors such as physician behavior and patient preferences may be related to the racial/ethnic differences and are key areas for future research [25], [26], [27].
The study informs our understanding of racial disparities in dementia risk. Studies using in-depth clinical examinations for dementia ascertainment reported mixed evidence on elevated risk of dementia for blacks, in geographically restricted samples [28], [29], [30], [31]. Using data from ADAMS, Plassman et al. [32] found no black-white difference in dementia risk, yet the estimation was based on a small sample of blacks. In this study, we observed higher rates of dementia prevalence for blacks and Hispanics than for whites based on either diagnosis in claims data or cognitive test in survey data.
After cognitive decline, college-educated individuals had lower odds of surviving without diagnosis than individuals with less than high school education. The college-educated were more likely assessed as having “normal” cognition after receiving a dementia diagnosis and to have a shorter lag between low cognition and diagnosis. These results are consistent with a cognitive reserve hypothesis [33], [34] contending that education would mitigate the symptoms of dementia, such as impaired cognition, until dementia is at a more advanced stage. Furthermore, highly educated individuals may be more likely to be diagnosed than individuals of lesser level of education as a result of better access to and utilization of health-care services. Several studies have called for an adjustment for education in cognitive tests [8], [35]. However, trade-offs between standardization of test and precision of estimation require further investigations.
Women were more likely than men to have agreement in prevalent dementia across measures consistent with empirical findings of a higher risk of dementia, which may be driven by genetic differences, social-cultural factors, or mortality selection [36], [37], [38], [39]. We did not find gender difference in disagreement across measures (i.e., cognitive tests only or diagnosis only).
Over time, we observed a potential improvement in diagnostic practice between 2000 and 2008, as shown by the shrinking likelihood of having prevalent “dementia by cognitive tests only,” coupled with that of having diagnosis more than 2 years after incident dementia based on cognitive tests. Continued efforts are needed to alleviate barriers to diagnosis, including increased access to care or improvement in physicians' knowledge about dementia and willingness to diagnose [3], especially for groups vulnerable to missed diagnosis. A timely diagnosis not only confers benefits to patients and families afflicted with dementia [40], [41], [42] but also reduces the cost of long-term care for the health-care system [43], [44].
There exist several limitations in this study. Although broadly representative, this sample does not include individuals in Medicare HMOs, who are more likely to be racial/ethnic minorities and younger [45] and only includes respondents consenting for linkage to Medicare claims who tend to be younger, non-white, and wealthier [46], [47]. Measurement error in ascertaining dementia is reduced by requiring a second dementia ascertainment and by examining change in cognition, rather than cross-sectional variations in cognition. However, some subtypes of dementia may manifest in symptoms that are not well detected by the set of cognitive tests in the HRS but may be diagnosed by a clinician. Measurement error may vary by race and education. For example, if cognitive batteries in the HRS are less sensitive to cognitive decline among highly educated individuals relative to those of lesser education level, these individuals would have a lower likelihood of being in our subsample analysis of incident diagnosis after cognitive decline and thus less likely to be at risk of “no diagnosis.” Similarly, non-whites may be more likely than whites to be categorized incorrectly with cognitive decline. Thus, disparities by education and race/ethnicity in the onset of dementia without diagnosis may be over-stated.
In conclusion, Medicare claims data yield equal prevalence estimates as nationally, representative survey data. These data are important data resources for researchers quantifying dementia in the US population and how it is changing over time. However, disparities in concordance of measures by race and education level shed light on data limitations in both survey and claims data. Blacks, Hispanics, and persons with low level of education are at risk of having no or delayed diagnosis. Using survey data containing cognitive tests to measure dementia may underidentify incidence among whites and college-educated persons. Methodological advances for identifying dementia by cognitive assessment in surveys are needed. Policy change such as inclusion of cognitive assessment in the new Medicare Annual Wellness Visit and reimbursement for this visit may be improving recognition of dementia in clinical practice and across diverse populations.
Research in context.
-
1.
Systematic review: Authors reviewed studies comparing US data sources for measuring dementia. Few studies assessed dementia prevalence, none incidence, comparing dementia diagnosis to cognitive assessment. Medicare claims may be a rich data source for tracking population dementia rates. Insufficient understanding of completeness of diagnosis, and for whom, limits their use.
-
2.
Interpretation: We improved methods for identifying dementia using longitudinal assessment and compared diagnosis in Medicare claims and survey-based cognitive tests. We linked individuals across data sources, over time, and found similar levels of prevalent dementia based on diagnosis and cognitive tests and identified populations with timely or delayed diagnoses.
-
3.
Future directions: The study identified strengths/weaknesses of diagnosis and survey-based dementia assessment for identifying dementia. The study proposes improved methods for identifying dementia, informs best use and caveats for future studies using Medicare claims, and identifies populations at risk of delayed diagnosis for policy or clinical interventions.
Acknowledgments
This work was funded by National Institutes of Health and National Institute on Aging (grant number: 1R01AG055401, 5P30AG024968, P30AG043073).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.04.003.
Supplementary data
References
- 1.Valcour V.G., Masaki K.H., Curb J.D., Blanchette P.L. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–2968. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- 2.Chodosh J., Petitti D.B., Elliott M., Hays R.D., Crooks V.C., Reuben D.B. Physician recognition of cognitive impairment: evaluating the need for improvement. J Am Geriatr Soc. 2004;52:1051–1059. doi: 10.1111/j.1532-5415.2004.52301.x. [DOI] [PubMed] [Google Scholar]
- 3.Bradford A., Kunik M.E., Schulz P., Williams S.P., Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23:306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa K.M., Larson E.B., Crimmins E.M., Faul J.D., Levine D.A., Kabeto M.U. A Comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zissimopoulos J., Crimmins E., St Clair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2014;18:25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zissimopoulos J., Tysinger B., S t.Clair P., Crimmins E. The impact of changes in population health and mortality on future prevalence of Alzheimer's disease and other dementias in the United States. J Gerontol B Psychol Sci Soc Sci. 2018;73:S38–S47. doi: 10.1093/geronb/gbx147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotwal A.A., Schumm L.P., Kern D.W., McClintock M.K., Waite L.J., Shega J.W. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Assoc Disord. 2015;29:317–324. doi: 10.1097/WAD.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spering C.C., Hobson V., Lucas J.A., Menon C.V., Hall J.R., O’Bryant S.E. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer's disease in ethnically diverse highly educated individuals: an analysis of the NACC database. J Gerontol A Biol Sci Med Sci. 2012;67:890–896. doi: 10.1093/gerona/gls006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knopman D.S., Roberts R.O., Geda Y.E., Pankratz V.S., Christianson T.J., Petersen R.C. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34:34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor D.H.Jr, Østbye T., Langa K.M., Weir D., Plassman B.L. The accuracy of medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E., Gatz M., Tseng C., Schneider L.S., Pawluczyk S., Wu A.H. Evaluation of medicare claims data as a tool to identify dementia. J Alzheimers Dis. 2019;67:769–778. doi: 10.3233/JAD-181005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor D.H., Jr., Fillenbaum G.G., Ezell M.E. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins C.H., Wilkins K.L., Meisel M., Depke M., Williams J., Edwards D.F. Dementia undiagnosed in poor older adults with functional impairment. J Am Geriatr Soc. 2007;55:1771–1776. doi: 10.1111/j.1532-5415.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson T., Ly A., Schnier C., Rannikmäe K., Bush K., Brayne C. Identifying dementia cases with routinely collected health data: A systematic review. Alzheimers Dement. 2018;14:1038–1051. doi: 10.1016/j.jalz.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopman D.S., Petersen R.C., Rocca W.A., Larson E.B., Ganguli M. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement. 2011;7:53–60. doi: 10.1016/j.jalz.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pressley J.C., Trott C., Tang M., Durkin M., Stern Y. Dementia in community-dwelling elderly patients: A comparison of survey data, medicare claims, cognitive screening, reported symptoms, and activity limitations. J Clin Epidemiol. 2003;56:896–905. doi: 10.1016/s0895-4356(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 17.Østbye T., Taylor D.H., Clipp E.C., Scoyoc L.V., Plassman B.L. Identification of dementia: agreement among national survey data, medicare claims, and death certificates. Health Serv Res. 2008;43:313–326. doi: 10.1111/j.1475-6773.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amjad H., Roth D.L., Sheehan O.C., Lyketsos C.G., Wolff J.L., Samus Q.M. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med. 2018;33:1131–1138. doi: 10.1007/s11606-018-4377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman V.A., Kasper J.D., Spillman B.C., Plassman B.L. Short-term changes in the prevalence of probable dementia: An analysis of the 2011-2015 National Health and Aging Trends Study. J Gerontol B Psychol Sci Soc Sci. 2018;73:S48–S56. doi: 10.1093/geronb/gbx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savva G.M., Arthur A. Who has undiagnosed dementia? A cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age Ageing. 2015;44:642–647. doi: 10.1093/ageing/afv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'fstedal M.B., Fisher G.G., Herzog A.R. 2005. Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor, MI: University of Michigan. [Google Scholar]
- 22.St Clair P., Gaudette É., Zhao H., Tysinger B., Seyedin R., Goldman D.P. Using Self-reports or claims to assess disease prevalence: It's complicated. Med Care. 2017;55:782–788. doi: 10.1097/MLR.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher G.G., Hassan H., Rodgers W.L., Weir D.R. 2015. Health and retirement study imputation of cognitive functioning measures: 1992–2010. Ann Arbor, MI: University of Michigan. [Google Scholar]
- 24.Crimmins E.M., Kim J.K., Langa K.M., Weir D.R. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill C.V., Pérez-Stable E.J., Anderson N.A., Bernard M.A. The National Institute on Aging health disparities research framework. Ethn Dis. 2015;25:245–254. doi: 10.18865/ed.25.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livney M.G., Clark C.M., Karlawish J.H., Cartmell S., Negrón M., Nuñez J. Ethnoracial differences in the clinical characteristics of Alzheimer's disease at initial presentation at an urban Alzheimer's disease center. Am J Geriatr Psychiatry. 2011;19:430–439. doi: 10.1097/JGP.0b013e3181f7d881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukadam N., Cooper C., Livingston G. Improving access to dementia services for people from minority ethnic groups. Curr Opin Psychiatry. 2013;26:409–414. doi: 10.1097/YCO.0b013e32835ee668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick A.L., Kuller L.H., Ives D.G., Lopez O.L., Jagust W., Breitner J.C. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 29.Hebert L.E., Bienias J.L., Aggarwal N.T., Wilson R.S., Bennett D.A., Shah R.A. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz M.J., Lipton R.B., Hall C.B., Zimmerman M.E., Sanders A.E., Verghese J. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M.X., Cross P., Andrews H., Jacobs D.M., Small S., Bell K. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 32.Plassman B.L., Langa K.M., McCammon R.J., Fisher G.G., Potter G.G., Burke J.R. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 34.Stern Y., Albert S., Tang M.X., Tsai W.Y. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 35.Crum R.M., Anthony J.C., Bassett S.S., Folstein M.F. Population-based norms for the Mini-mental state examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 36.Azad N.A., Al Bugami M., Loy-English I. Gender differences in dementia risk factors. Gend Med. 2007;4:120–129. doi: 10.1016/s1550-8579(07)80026-x. [DOI] [PubMed] [Google Scholar]
- 37.Andersen K., Launer L.J., Dewey M.E., Letenneur L., Ott A., Copeland J.R.M. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 38.Ruitenberg A., Ott A., van Swieten J.C., Hofman A., Breteler M.M. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22:575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 39.Mielke M.M., Vemuri P., Rocca W.A. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett J.H., Lewis L., Blackwell A.D., Taylor M. Early intervention in Alzheimer's disease: a health economic study of the effects of diagnostic timing. BMC Neurol. 2014;14:101. doi: 10.1186/1471-2377-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borson S., Frank L., Bayley P.J., Boustani M., Dean M., Lin P.J. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9:151–159. doi: 10.1016/j.jalz.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carpenter B.D., Xiong C., Porensky E.K., Lee M.M., Brown P.J., Coats M. Reaction to a dementia diagnosis in individuals with Alzheimer's disease and mild cognitive impairment. J Am Geriatr Soc. 2008;56:405–412. doi: 10.1111/j.1532-5415.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 43.Long K.H., Moriarty J.P., Mittelman M.S., Foldes S.S. Estimating the potential cost savings from the New York University Caregiver Intervention in Minnesota. Health Aff (Millwood) 2014;33:596–604. doi: 10.1377/hlthaff.2013.1257. [DOI] [PubMed] [Google Scholar]
- 44.Weimer D.L., Sager M.A. Early identification and treatment of Alzheimer's disease: social and fiscal outcomes. Alzheimers Dement. 2009;5:215–226. doi: 10.1016/j.jalz.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuire T.G., Newhouse J.P., Sinaiko A.D. An economic history of Medicare part C. Milbank Q. 2011;89:289–332. doi: 10.1111/j.1468-0009.2011.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakshaug J.W., Weir D.R., Nicholas L.H. Identifying diabetics in Medicare claims and survey data: implications for health services research. BMC Health Serv Res. 2014;14:150. doi: 10.1186/1472-6963-14-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sala E., Knies G., Burton J. Propensity to consent to data linkage: experimental evidence on the role of three survey design features in a UK longitudinal panel. Int J Social Res Methodol. 2014;17:455–473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.