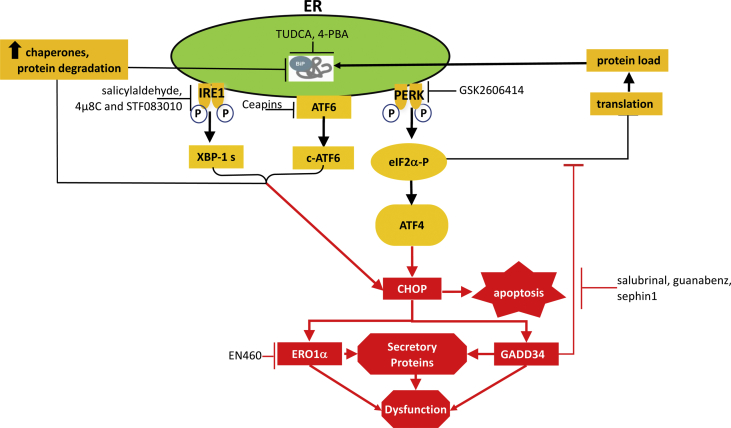
Fig. 1.
Rationale underlying the targeting of ER stress and the ER stress response.
Unfolded proteins in the ER cause ER stress and activate an ER stress response triggered by three sensors (IRE1, PERK and ATF6) which, together, attenuate protein synthesis and thus limit organelle load and promote the transcriptional up-regulation of the genes encoding the components of the ER protein handling machinery. This adaptive response is aimed at reducing ER stress and restoring ER homeostasis (black arrows). However, there is also a maladaptive response (red arrows) because the IRE1, ATF6 and PERK pathways are also connected to pro-apoptotic signals via the CHOP transcription factor (GADD153), which promotes the recovery of translation and oxidative protein folding by inducing GADD34 and ERO1 respectively, a process that leads to death due to the accumulation of malfolded proteins and the generation of excessive reactive oxygen species. Various inhibitors of ER stress and the ER stress response are available: the chemical chaperones TUDCA and 4-PBA help protein folding; salicylaldehydes, 4μ8C and STF083010 inhibit the endonuclease activity of IRE1-alpha; GSK2606414 inhibits the kinase activity of PERK; and the ceapin class of molecules inhibits the trafficking of ATF6-alpha to the nucleus by trapping it inside the ER. EN460 inhibits ERO1 alpha activity and salubrinal, gunabenz and sephin1 inhibit GADD34 activity.