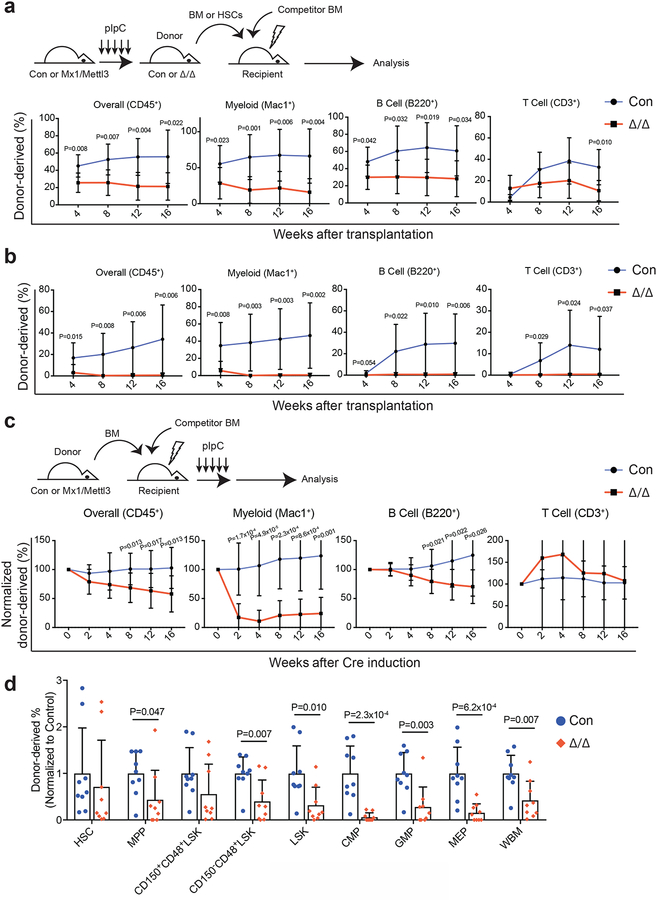
Figure 3: Loss of Mettl3 disrupts HSC differentiation in vivo.
(a) Competitive transplantation of 500,000 donor whole bone marrow cells from pIpC-treated Mx1-cre; Mettl3fl/fl or Mettl3fl/fl controls with 500,000 competitor cells. Multi-lineage chimera levels in the peripheral blood were assessed up to 16 weeks after transplantation (n=7 for control, n=8 for Mx1-cre; Mettl3fl/fl; samples were from independent recipients of two independent donor pairs from two experiments).
(b) Competitive transplantation of 50 sorted HSCs from pIpC-treated Mx1-cre; Mettl3fl/fl or controls with 300,000 competitor cells. Multi-lineage chimera levels in the peripheral blood were assessed up to 16 weeks after transplantation (n=9 for control, n=9 for Mx1-cre; Mettl3fl/fl; samples were from independent recipients of two independent donor pairs from two experiments).
(c) 500,000 donor whole bone marrow cells from untreated Mx1-cre; Mettl3fl/fl or controls with 500,000 competitor cells were transplanted into lethally irradiated recipient mice. Recipients were treated with pIpC after stable peripheral blood chimerism was established. Multi-lineage peripheral blood chimera levels shown as the percentage of the original chimera level up to 16 weeks after pIpC treatment (n=11 for control, n=12 for Mx1-cre; Mettl3fl/fl; samples were from independent recipients of three independent donor pairs from three experiments).
(d) Chimera levels of indicated bone marrow cell populations 20 weeks after pIpC treatment from (c) (n=9 for control, n=9 for Mx1-cre; Mettl3fl/fl; samples were from independent recipients of three independent donor pairs from three experiments).
Values are shown as individual points with mean ± s.d. P values were determined by unpaired two-sided Student’s t-test.