Scheme 1.
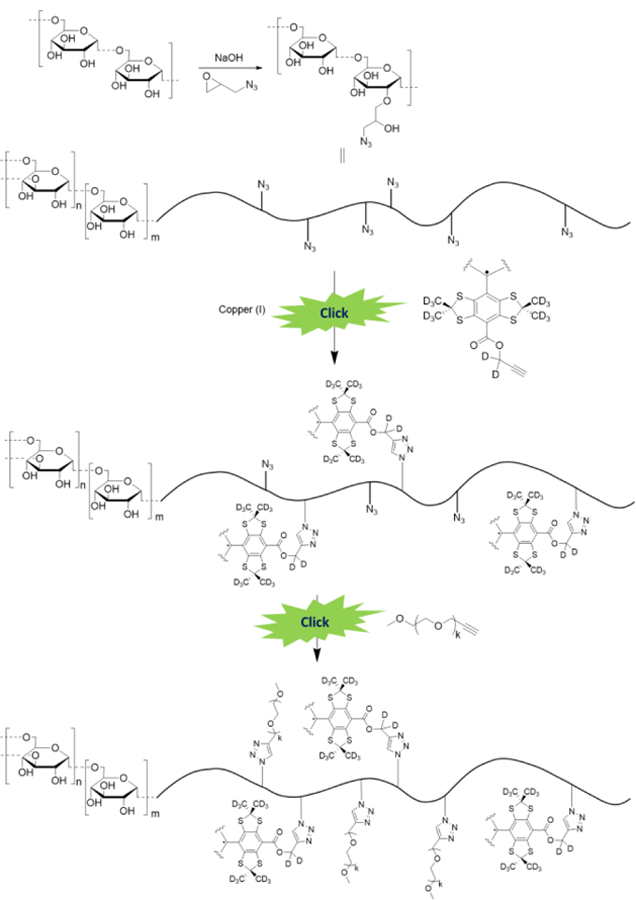
Schematic representation of the synthesis of dextran-conjugated TAM radicals using a click chemistry approach. The dextran grafting by the dFTr radical was achieved by copper-catalyzed azide-alkyne cycloaddition (CuAAC) of a TAM mono-propargyl ester followed by addition of excess of alkyne-PEG. Varying the trityl radical/dextran ratio allows for the tuning of the radical loading onto the polymer. Incorporation of PEG chains enhances the solubility of the grafted dextran and prevents spin-spin interactions between trityl radicals.
