Abstract
Rationale:
Complement activation contributes to multiple immune-mediated pathologies. In late allograft failure, donor-specific antibody deposits complement membrane attack complexes (MAC) on graft endothelial cells (ECs), substantially increasing their immunogenicity without causing lysis. Internalized MAC stabilize NF-κB–inducing kinase (NIK) protein on Rab5+MAC+ endosomes, activating non-canonical NF-κB signaling. However, the link to increased immunogenicity is unclear.
Objective:
To identify mechanisms by which alloantibody and internalized MAC activate ECs to enhance their ability to increase T cell responses.
Methods and Results:
In human EC cultures, internalized MAC also causes NLRP3 translocation from endoplasmic reticulum to Rab5+MAC+NIK+ endosomes followed by endosomal NIK-dependent inflammasome assembly. Cytosolic NIK, stabilized by LIGHT, does not trigger inflammasome assembly and ATP-triggered inflammasome assembly does not require NIK. IFN-γ primes EC responsiveness to MAC by increasing NLRP3, pro-caspase 1 and gasdermin D expression. NIK-activated non-canonical NF-κB signaling induces pro-IL-1β expression. Inflammasome processed pro-IL-1β and gasdermin D results in IL-1β secretion that increases EC immunogenicity through IL-1R signaling. Activation of human ECs lining human coronary artery grafts in immunodeficient mouse hosts by alloantibody and complement similarly depends upon assembly of an NLRP3 inflammasome. Finally, in renal allograft biopsies showing chronic rejection, caspase-1 is activated in C4d+ ECs of interstitial microvessels, supporting the relevance of the cell culture findings.
Conclusions:
In response to antibody-mediated complement activation, IFN-γ-primed human endothelial cells internalize MAC, triggering both endosomal-associated NIK-dependent NLRP3 inflammasome assembly and IL-1 synthesis resulting in autocrine/paracrine IL-1β-mediated increases in EC immunogenicity. Similar responses may underlie other complement-mediated pathologies.
Subject Terms: Cell Signaling/Signal Transduction, Inflammation, Mechanisms, Transplantation, Vascular Biology
Keywords: Endothelium, transplantation, immune system, complement, inflammasome, memory T cells
INTRODUCTION
Vascular endothelium performs critical homeostatic functions, including direct participation in protective innate and adaptive immune responses. Specifically, activation of endothelial cells (ECs) by TNF or IL-1 targets recruitment of circulating leukocytes to sites of infection1. Circulating IFN-γ induces human ECs express both class I and class II human leukocyte antigen (HLA) molecules as well as various co-stimulators that allow microbial antigen presentation to circulating effector memory CD8+ and CD4+ T cells, respectively, specifically targeting T cell extravasation to sites of infection1, 2. Allograft rejection is an unintended consequence of these protective functions as the immune system responds to cells expressing non-self HLA molecules as if they were infected. B cells also respond to allografts, producing complement-fixing donor-specific antibodies (DSA) that primarily react with non-self HLA molecules on graft ECs3. Antibody binding to vascular ECs is associated with tissue injury in multiple disease settings and is a specific hallmark of late graft loss due to chronic rejection4. The binding of DSA to ECs activates complement and deposits membrane attack complexes (MAC) on the luminal EC surface. Remarkably, this occurs without evidence of EC lysis and instead mimics the effects of inflammatory cytokines, resulting in EC activation5. These changes further augment the alloreactive T cell response, linking humoral and cell-mediated alloimmunity5. Chronic rejection of solid organ transplants presents a human model for studying how these processes may mediate other complement-dependent manifestations of chronic tissue inflammation and injury. However, the mechanism(s) underlying how MAC potentiates the immunogenicity of ECs, measured as effector memory T cell proliferation and cytokine (especially IFN-γ) production is incompletely understood.
In prior studies using cultured human ECs and complement-fixing alloantibodies from sera of pre-sensitized transplant candidates with high panel reactive antibody (PRA) titers, we observed that MAC deposited on human ECs is rapidly internalized through a clathrin-mediated pathway and leads to post-translational stabilization of NF-κB-inducing kinase (NIK) on the surface of Rab5+MAC+ early endosomes within 30 minutes5, 6. Endosome-associated NIK initiates non-canonical NF-κB activation, indicated by p100 proteolytic conversion to p52 and p52/RelB translocation to the nucleus. This is followed in a few hours by an induction of proinflammatory genes characteristic of cytokine-mediated EC activation including expression of adhesion molecules, chemokines and cytokines. These responses were linked to NIK by the observation that siRNA-mediated knockdown of either NIK or p100 prevented complement-mediated activation of the EC5. MAC-induced changes in the ECs of a graft explain increased T cell recruitment5, 6.
Here we further investigate the mechanisms linking endosomal NIK to potentiated EC immunogenicity. We report that internalized MAC additionally and rapidly triggers NLRP3 inflammasome assembly in a process initiated by endosome-associated but not cytosolic NIK. This endosome-based response is not required for adenosine triphosphate (ATP)-induced assembly of an NLRP3 inflammasome. It also differs from inflammasomes assembled in response to pore-forming molecules, such as aerolysin, in that IPAF (NLRC4) is not involved7. Upon MAC internalization, the inflammasome sensor protein NLRP3 translocates from the endoplasmic reticulum to Rab5+MAC+NIK+ endosomes followed by recruitment of the adaptor protein ASC and pro-caspase-1, assembling into an inflammasome that autocatalytically activates caspase-1. This process is primed by IFN-γ, which upregulates the expression of both NLRP3 and pro-caspase-1 as well as gasdermin D (GSDMD). IFN-γ also induces pro-IL-1β, but this effect is very transient. However, pro-IL-1β is stably and more effectively induced by NIK through non-canonical NF-κB activation, explaining the observation that p100 knockdown abrogated complement-mediated EC activation and enhanced T cell activation and proliferation. The inflammasome then converts both pro-IL-1β and the pore-forming protein GSDMD to their active forms, resulting in secretion of mature IL-1β. The activation of ECs follows in an autocrine/paracrine manner that can be inhibited by IL-1 receptor antagonist (IL-1Ra). These findings further elucidate a novel pathway by which antibody and internalized MAC activate ECs, leading to augmented T cell responses and tissue injury without inducing lysis of the ECs and further suggests new potential therapeutic targets for reducing graft loss due to chronic rejection. Our findings may also have much broader applicability to other complement-associated pathologies, examples being systemic lupus erythematosus, rheumatoid arthritis and ischemia reperfusion injury.
METHODS
This manuscript adheres to the Transparency and Openness Promotion Guidelines of the American Heart Association. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Detailed experimental procedures are described in the Materials and Methods section of the online-only Supplementary Materials. All protocols using human materials were approved by the relevant Institutional Review Boards and those involving animals by the Yale Institutional Animal Care and Use Committee. In vitro studies of EC responses to complement were elicited using discarded and pooled preparations of high titer PRA sera obtained from the Yale HLA tissue typing lab to treat serially passaged human umbilical vein ECs (HUVECs) cultures. Studies of memory T cell responses were assayed in co-cultures of peripheral blood human CD4+CD45RO+HLA-DR- T lymphocytes with allogeneic HUVECs. In vitro findings were confirmed in human artery xenografts in immunodeficient mice and in de-identified renal biopsies. Methodologies include immunofluorescence microscopy, flow cytometry, western blotting, reporter genes, quantitative RT-PCR and ELISA.
RESULTS
Alloantibody-activated and internalized MAC activate an NLRP3 inflammasome in IFN-γ-pretreated human ECs.
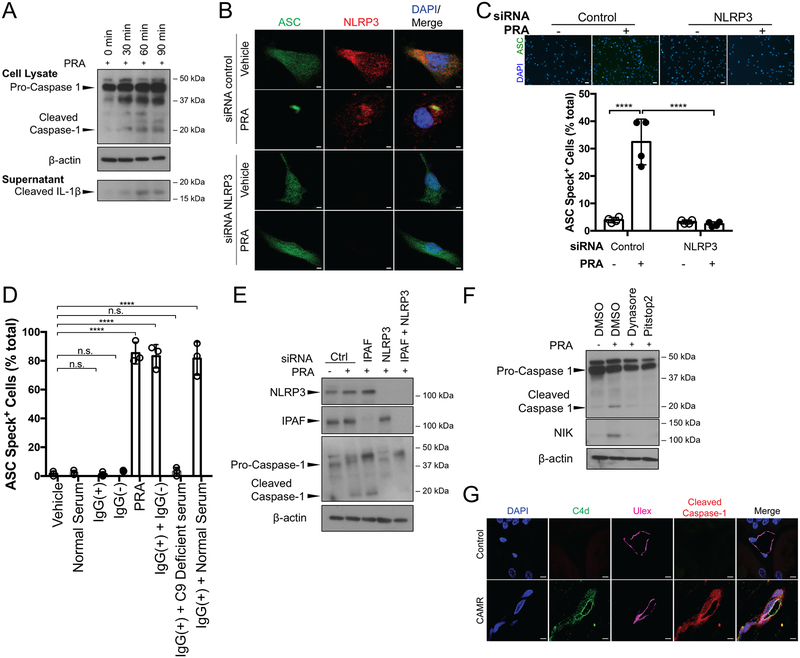
Binding of complement-fixing human alloantibodies present in high titer PRA sera to cultured human ECs in GVB buffer that allows complement activation leads to formation and rapid internalization of MAC without causing cell lysis5, 6. Internalized MAC activates a signaling pathway that leads to rapid processing and nuclear translocation of p52/RelB, detectable within 30 minutes5, 6. Although human graft vessel ECs constitutively express both class I and II HLA molecules in situ, cultured ECs reduce their expression of class I molecules and completely lose expression of class II molecules, presumably due to the absence of human IFN-γ under standard culture conditions8. Expression of HLA molecules, which are the molecular targets of alloantibody in PRA, can be restored by human IFN-γ treatment, significantly increasing both PRA binding and complement activation5, 6. Here, we show PRA binding and complement activation of IFN-γ-pretreated ECs concomitantly results in the assembly of an NLRP3 inflammasome and secretion of IL-1β protein (Figure 1A, Supplemental Figure IA). Inflammasome activation, detected by immunoblot analysis of proteolytically processed caspase-1 in cell lysates and processed IL-1β in culture medium, can be seen within 30 minutes of PRA-induced complement activation. To confirm these results, ECs were transduced to over-express an ASC-green fluorescent protein (GFP) fusion construct (ASC-GFP) so that oligomerization of ASC-GFP can then be detected as a single fluorescent “speck” and much, but not all, immunofluorescence staining of NLRP3 colocalized with the assembled specks (Figure 1B). siRNA-mediated knockdown of NLRP3 inhibited ASC oligomerization (Figure 1B and 1C), identifying NLRP3 as the sensor that initiates inflammasome assembly. To determine what component(s) of the PRA serum are necessary for inflammasome formation, PRA serum was separated into IgG(+) and IgG(−) fractions. ASC speck formation was not detected in response to either the antibody-containing IgG(+) fraction or the complement-containing IgG(−) fraction when added separately to ECs (Figure 1D, Supplemental Figure IB). Activity was regained when the two fractions were recombined. Whole human serum controls lacking anti-HLA antibodies also failed to trigger speck formation but ASC speck formation was detected when purified IgG from PRA sera was combined with complement-containing whole serum from an unrelated donor but not when combined with C9-deficient serum, suggesting that MAC is essential for inflammasome activation.
Figure 1. Alloantibody-mediated MAC deposition on IFN-γ-pretreated human ECs activates an NLRP3 inflammasome.
(A) ECs pre-treated with IFN-γ (50ng/mL) for 48 hours prior to PRA sera treatment for the indicated times and whole cell lysates and culture supernatants were assessed for cleaved caspase-1 and cleaved IL-1β, respectively, by immunoblot. (B) ASC-GFP transduced and IFN-γ pre-treated ECs were transfected with control or NLRP3 siRNA and treated with PRA sera prior to confocal microscopy staining for NLRP3. Scale bar, 2 μm. (C) Top: ASC speck formation of control and NLRP3 siRNA transfected PRA-treated ASC-GFP ECs were assessed by epifluorescence. Scale bar, 50 μm. Bottom: Each symbol represents the mean of the % ASC speck+ cells in 3 low powered fields per sample (n=4, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (D) PRA sera was separated into IgG(+) and IgG(−) fractions and added to IFN-γ pre-treated ASC-GFP ECs either alone, or in combination with C9 deficient or normal serum as indicated for ASC speck formation analysis. Each symbol represents the mean of the % ASC speck+ cells in at least 3 low powered fields per sample. (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (E) IFN-γ pre-treated ECs were transfected with either control, IPAF, NLRP3 or both IPAF and NLRP3 siRNA prior to treatment with PRA and cell lysates were assessed for cleaved caspase-1 by immunoblot. (F) IFN-γ-primed ECs were pre-treated with dynasore or pitstop2, two pharmacological inhibitors of clathrin-mediated endocytosis, treated with PRA sera for 30 minutes. Whole cell lysates were analyzed for cleaved caspase-1 and NIK by immunoblot. (G) Renal biopsies from patients with chronic antibody mediated rejection (CAMR) or taken at the time of transplant (control) were co-stained for C4d, Ulex and cleaved caspase-1 and analyzed by confocal microscopy. Scale bar, 5 μm. ****P<0.0001, n.s., non-significant.
Aerolysin is a pore-forming bacterial toxin that has been shown to assemble both NLRP3 and IPAF (also known as NLRC4) inflammasomes7. This prompted us to test whether MAC, a pore-forming protein complex, utilized one or both of these inflammasome sensor molecules. Complement-mediated activation of caspase-1, detected by western blotting, was inhibited by siRNA knockdown of NLRP3, confirming the speck assay results, but not by siRNA knockdown of IPAF (Figure 1E, Supplemental Figure IC), suggesting differential signaling pathway(s) induced by MAC compared to aerolysin. In our prior studies, MAC-induced activation of non-canonical NF-κB required its internalization by a clathrin-mediated process, resulting in initiation of signaling on Rab5+MAC+ endosomes and not at the cell surface. We pre-treated ECs with two different inhibitors of clathrin-mediated endocytosis, dynasore and pitstop2, and found that these agents blocked inflammasome assembly (Figure 1F, Supplemental Figure ID). This indicated that pore formation by MAC at the cell surface was insufficient to induce inflammasome assembly and that signal(s) derived intracellularly were instead required. Together, our data demonstrate that alloantibody-induced MAC deposition on ECs requires internalization to assemble an NLRP3 inflammasome and release of IL-1β.
Renal allograft biopsies from patients, who have developed a complement-fixing DSA and are diagnosed with chronic antibody-mediated rejection (CAMR), show C4d deposition on ECs lining interstitial capillaries9, 10. To test whether inflammasome activation occurs in human graft ECs, we used immunofluorescence to analyze renal allograft biopsies from patients with graft dysfunction in the setting of DSA and with evidence of C4d deposition on peritubular capillary ECs. In these biopsies, cleaved caspase-1 colocalized with EC staining for C4d. Neither C4d nor cleaved caspase-1 were observed in time zero control renal biopsies (Figure 1G). These data indicate that caspase-1 activation occurs in ECs concomitant with complement deposition in renal CAMR, consistent with our in vitro experiments.
IFN-γ primes human ECs for MAC-induced NLRP3 inflammasome activation.
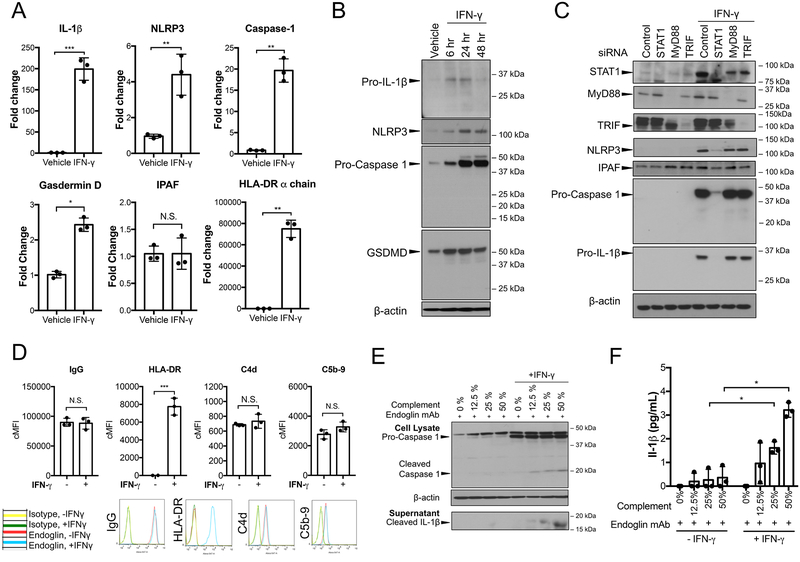
Secretion of active IL-1β is dependent upon synthesis of the pro-protein form of the cytokine as well as processing of the pro-protein by an assembled inflammasome11. In murine macrophages, pro-protein synthesis is initiated at the transcriptional level by engagement of pattern recognition receptors (PRRs) that signal through the MyD88 adaptor protein and is dependent upon canonical NF-κB activation12, 13. In our cell culture model, human ECs are not exposed to ligands for pattern recognition receptors and under basal culture conditions, there is no evidence of canonical NF-κB signaling 30 minutes after MAC deposition. However, the cells are pretreated with IFN-γ to re-induce HLA molecules, thereby increasing alloantibody binding and complement activation. We wondered if there might be a role for IFN-γ in priming inflammasome components. Interestingly, IFN-γ treatment alone increased NLRP3, caspase-1, IL-1β and GSDMD transcript and protein levels (Figure 2A and 2B, Supplemental Figure IIA). However, IFN-γ induction of pro-IL-1β protein was transient and waned considerably at 48 hours, while inductions of inflammasome components NLRP3 and pro-caspase-1 and of GSDMD protein were sustained (Figure 2B, Supplemental Figure IIA). As shown by siRNA knockdowns, IFN-γ priming is independent of MyD88 and TRIF, transcription factors downstream of PRR priming, and instead dependent on STAT1 (Figure 2C, Supplemental Figure IIB). IFN-γ did not enhance IPAF transcript or protein levels (Figure 2A and C, Supplemental Figure IIB). To assess the extent to which MAC-dependent inflammasome assembly and secretion of IL-1β require IFN-γ priming, we used an anti-human endoglin mouse mAb that bound equally to IFN-γ-primed and unprimed human ECs and deposited equivalent amounts of human MAC on the surface of unprimed and IFN-γ primed ECs (Figure 2D). Human serum was titrated to vary the level of complement deposition and ECs were monitored for both caspase-1 activation and IL-1β secretion. Induction of pro-caspase-1 protein and caspase-1 activation was much greater in human ECs primed with IFN-γ (Figure 2E, Supplemental Figure IIC) and increasing the level of MAC deposition correlated directly with increased detection of cleaved IL-1β in the supernatant, as detected by immunoblot or measured by ELISA (Figure 2E and 2F). Indeed, both inflammasome activation and IL-1β secretion were minimal in unprimed cells (Figure 2E and F). These results collectively suggest that IFN-γ not only regulates HLA antigen expression but also primes human ECs for NLRP3 inflammasome formation and IL-1β production and maturation, replacing the need for priming by engagement of PRRs. Since high levels of HLA class I and especially class II are expressed on human ECs in situ, it is likely that human ECs are basally primed for NLRP3 inflammasome assembly but not for expression of pro-IL-1β.
Figure 2. IFN-γ primes human ECs for MAC-induced NLRP3 inflammasome activation.
(A) ECs were primed with IFN-γ (50ng/mL) for 24 hours and upregulation of IL-1β, NLRP3, NLRC4/IPAF, Caspase-1, Gasdermin D and HLA-DR α-chain transcript levels were assessed by qRT-PCR (n=3, Student’s t-test, SEM). (B) ECs were stimulated with IFN-γ for the duration indicated and pro-IL-1β, NLRP3, pro-caspase-1, and gasdermin D expression were assessed by immunoblot. (C) Unprimed and IFN-γ-primed ECs were transfected with control, STAT1, MyD88, or TRIF siRNA and protein expression of NLRP3, IPAF, pro-caspase-1 and pro-IL-1β were assessed by immunoblot. (D) Unprimed and IFN-γ-primed ECs were incubated with complement-fixing anti-human endoglin IgG2a mouse monoclonal antibody (mAb) (20μg/mL) and human complement prior to flow cytometry analysis of IgG binding, HLA-DR, C4d and C5b-9 (n=3, Student’s t-test, SEM). (E) Unprimed and IFN-γ-primed ECs were incubated with anti-human endoglin IgG2a mAb and varying concentrations of human complement. Cell lysates and culture supernatants were assessed for cleaved caspase-1 and cleaved IL-1β, respectively, by immunoblot. (F) ELISA measurement of IL-1β secretion in culture supernatants by unprimed and IFN-γ-primed ECs after incubation with anti-human endoglin mAb and varying levels of human complement (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). *P<0.05, **P<0.01, ***P<0.001, n.s., non-significant.
Dual role of Rab5+ endosome-associated NIK in non-canonical NF-κB activation and inflammasome assembly for sustained pro-IL-1β expression and processing, respectively.
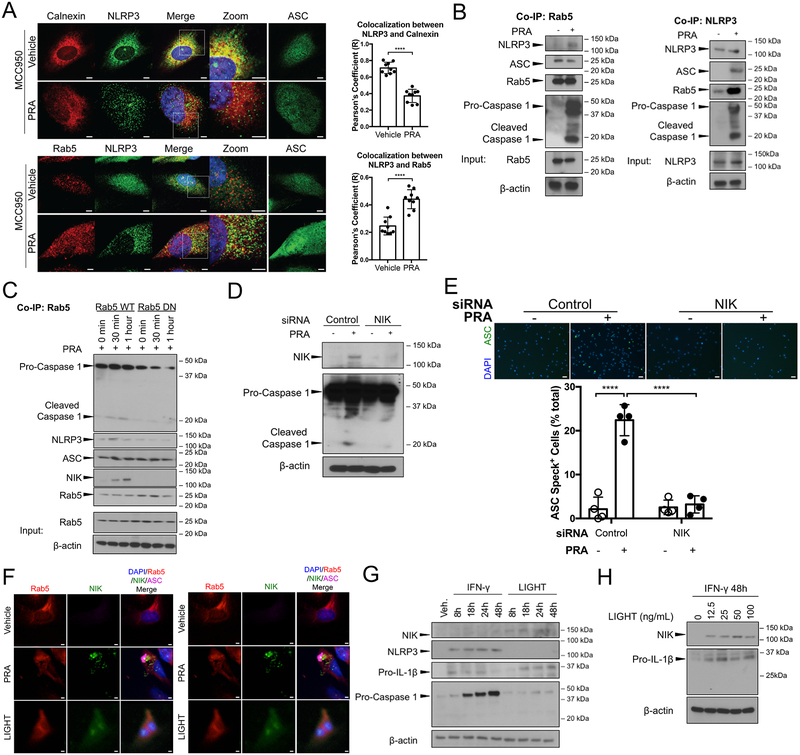
We next turned to identifying signaling pathway(s) activated by MAC that lead to inflammasome assembly. As shown above (Figure 1F), MAC-mediated assembly of the inflammasome required clathrin-mediated endocytosis. In our prior studies of non-canonical NF-κB activation, we found that MAC internalized by clathrin-mediated endocytosis is transferred to early Rab5+ endosomes, which undergo remodeling and become signaling platforms that stabilize newly translated NIK protein. We therefore examined the role of Rab5+MAC+ endosomes in inflammasome assembly. In murine macrophages, resting NLRP3 and its adaptor ASC have been observed to colocalize with endoplasmic reticulum and cytosol, respectively, and ATP is a potent co-activator of the NLRP3 inflammasome14, 15. The localization of the inflammasome sensor NLRP3 in untreated, PRA-treated and ATP-treated human ECs was examined using confocal microscopy. As described above, PRA activation of complement rapidly induces speck formation, which could obscure early signal events. To focus on the latter, we pretreated ASC-GFP transduced ECs with MCC950, a drug that blocks the association of NLRP3 with ASC16. Under non-stimulatory conditions, NLRP3 protein was found to mostly localize to perinuclear granular structures and significant colocalization was found with the endoplasmic reticulum (ER) marker calnexin. Very little or no colocalization was detected with Rab5 or with mitochondria marker COX IV, Golgi marker GOPC, or lysosome marker LAMP1 (Figure 3A, Supplemental Figure IIIA). Upon stimulation with PRA, NLRP3 rapidly translocated to Rab5+ structures (Figure 3A). We confirmed that PRA treatment led to increased association of NLRP3 with Rab5+ endosomes by co-immunoprecipitations (co-IP) of Rab5 and NLRP3 (Figure 3B, Supplemental Figure IIIB). While ATP also triggers speck formation and caspase-1 activation, ATP-induced inflammasome formation does not lead to NLRP3 translocation to Rab5 endosomes and does not require NIK (Supplemental Figure IIIC, IIID and IIIE). These data indicated that MAC-triggered assembly of NLRP3 inflammasomes, unlike that of ATP-triggered inflammasomes, is initiated on Rab5+ endosomes. To determine if Rab5 GTPase activity, required for NIK binding and stabilization, is also required for NLRP3 inflammasome assembly and activation, ECs were transfected with a Rab5 dominant negative (DN) construct or Rab5 wildtype (WT) construct. In ECs transfected with Rab5 WT and then treated with PRA, cleaved caspase-1 and NIK coimmunoprecipitated with Rab5 but did not do so in Rab5 DN ECs, indicating that Rab5 activity is necessary for inflammasome assembly and activation (Figure 3C, Supplemental Figure IIIF). Moreover, siRNA-mediated knockdown of NIK completely abrogated caspase-1 activation and ASC speck formation (Figure IIID and IIIE, Supplemental Figure IIIG).
Figure 3. Dual role of Rab5+ endosome-associated NIK in non-canonical NF-κB activation and inflammasome assembly for sustained pro-IL-1β expression and processing, respectively.
(A) Left: Confocal imaging analysis of IFN-γ-primed ASC-GFP ECs that were pretreated with NLRP3 inhibitor MCC950 to prevent ASC oligomerization or control DMSO prior to PRA sera treatment for 30 minutes and co-staining with NLRP3 and Rab5 or calnexin. Scale bar, 5 μm. Right: Pearson’s colocalization coefficients (R) were calculated for calnexin and NLRP3 (top) and Rab5 and NLRP3 (bottom). Each symbol represents a colocalization coefficient R calculated of ECs (n=9, Student t-test, SEM). (B) Rab5 and NLRP3 co-immunoprecipitations (co-IPs) were performed on PRA sera or vehicle treated IFN-γ-primed ECs and immunoblotted as indicated. (C) Stably transduced Rab5-WT or Rab5 DN (S43N) IFN-γ-primed ECs were treated with PRA sera prior to co-immunoprecipitation of Rab5 and subsequent immunoblot analysis for cleaved caspase-1, NLRP3, ASC and NIK. (D) IFN-γ-primed ECs were transfected with control or NIK siRNA and whole cell lysates were analyzed for cleaved caspase-1 and NIK by immunoblot. (E) Top: ASC speck formation in control and NIK siRNA transfected IFN-γ-primed ECs was assessed by epifluorescence. Scale bar, 50 μm. Bottom: Each symbol represents the mean of the percentage of ASC speck+ cells in at least 3 low powered fields per sample (n=4, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (F) Left: ASC-GFP transduced ECs were treated with either PRA sera or LIGHT (100ng/mL) and co-stained for Rab5 and NIK. Scale bar, 5 μm. Right: Confocal imaging analysis of ASC-GFP transduced ECs treated with either PRA sera or LIGHT, prior to RelB staining. Scale bar, 5 μm. (G) ECs were treated with either IFN-γ or LIGHT for 8, 18, 24 or 48 hours and cell lysates were analyzed for NIK, NLRP3, pro-IL-1β, pro-caspase-1 expression by immunoblot. (H) ECs were primed with IFN-γ for 48 hours and subsequently treated with varying concentrations of LIGHT for 18 hours prior to immunoblot analysis of NIK and pro-IL-1β expression. ****P<0.0001.
We next compared the effects of NIK stabilization by MAC, a process requiring Rab5+ endosomes, to that of LIGHT, a TNF superfamily member cytokine that activates non-canonical NF-κB signaling by stabilizing NIK in the cytosol in a process that does not involve Rab5+ endosomes. As expected, NIK stabilization following treatment with LIGHT resulted in diffuse cytosolic NIK staining, which contrasted with punctate NIK staining colocalizing with Rab5 following PRA treatment (Figure 3F, left). Also, as expected, LIGHT induced RelB nuclear translocation but did not induce ASC speck formation, unlike PRA sera, which induced both RelB nuclear translocation and ASC specks (Figure 3F, right). These data collectively indicated that, in response to MAC, Rab5 activity was required to sequester NIK protein on Rab5+ endosomes. Endosome-associated NIK subsequently elicited transfer of NLRP3 sensor protein from the ER to these same Rab5+ endosomes, followed by recruitment of ASC and caspase-1 effector molecules for terminal inflammasome activation.
Our new data raised the question as to whether non-canonical NF-κB signaling plays any role in IL-1β synthesis by MAC-activated ECs. As shown in Figure 2B, we had observed that while IFN-γ priming consistently led to transient induction of pro-IL-1β, expression of this pro-protein largely had waned at 48 hours in contrast to the sustained induction of inflammasome components NLRP3 and pro-caspase-1. In contrast to its failure to induce inflammasome assembly, LIGHT activation of non-canonical NF-κB led to markedly increased pro-IL-1β expression. LIGHT treatment did not affect NLRP3 or pro-caspase-1 expression (Figure 3G, Supplemental Figure IIIH). These data suggested that complement-activated non-canonical NF-κB signaling likely contributes to IL-1β secretion by inducing sustained pro-IL-1β expression. To test this, we primed ECs with IFN-γ for 48h, a time point at which pro-IL-1β expression had declined, and then initiated LIGHT treatment. We observed potent re-induction of pro-IL-1β (Figure 3H, Supplemental Figure IIII). These data indicate a dual role for endosome-associated NIK. Through non-canonical NF-κB signaling, NIK increases and sustains pro-IL-1β expression levels and independently initiates inflammasome assembly via an endosome-associated pathway. These responses combine to mediate IL-1β processing and secretion.
MAC-induced NLRP3 inflammasome induces IL-1β maturation and gasdermin-D-dependent IL-1β release from human ECs.
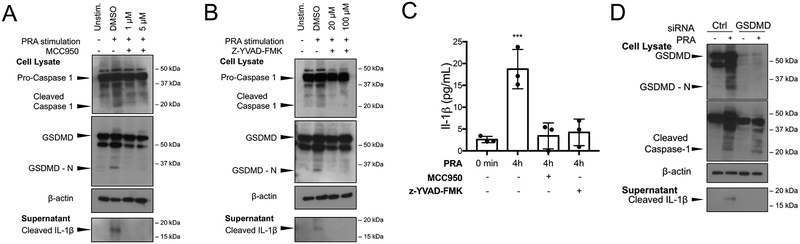
GSDMD is cleaved by caspase-1, releasing autoinhibition by the carboxy terminal region (GSDMD-C) on the amino terminal region (GSDMD-N) that then undergoes oligomerization with pore formation on the plasma membrane17. While excessive activation of this response can lead to cell death by pyroptosis, more limited activation is necessary and sufficient for release (“secretion”) of processed IL-1β and certain other cytokines18. As noted above in Figure 2A and 2B, IFN-γ priming increased expression of GSDMD. Assembly of an NLRP3 inflammasome following MAC deposition on primed EC resulted in processing of GSDMD to GSDMD-N, a process that was inhibited by either pharmacological inhibition of NLRP3 with MCC950 (Figure 4A, Supplemental Figure IVA) or of caspase-1 with z-YVAD-FMK (Figure 4B, Supplemental Figure IVB). Both inhibitors also prevented processed IL-1β release in the culture medium from PRA-treated ECs, detected by immunoblot (Figure 4A and 4B) or quantified by ELISA (Figure 4C). Furthermore, siRNA-mediated knockdown of GSDMD prevented cleaved IL-1β secretion but not caspase-1 activation (Figure 4D, Supplemental Figure IVC). These data indicate that the MAC-induced NLRP3 inflammasome formed in ECs following internalization of MAC induces processing of GSDMD to GSDMD-N, which then facilitates IL-1β release.
Figure 4. MAC-induced NLRP3 inflammasome induces IL-1β maturation and gasdermin-D-dependent IL-1β release from human ECs.
(A) IFN-γ-primed ECs were pre-treated with NLRP3 inhibitor MCC950 prior to PRA sera treatment and immunoblot analysis of whole cell lysates for cleaved caspase-1 and gasdermin D amino terminal region (GSDMD-N) and culture supernatants for cleaved IL-1β. (B) IFN-γ-primed ECs were pretreated with selective caspase-1 inhibitor z-YVAD-FMK prior to PRA sera treatment and immunoblot analysis of whole cell lysates for cleaved caspase-1 and GSDMD-N and culture supernatants for cleaved IL-1β. (C) ELISA measurements of IL-1β in culture supernatants of IFN-γ-primed ECs pretreated with z-YVAD-FMK or DMSO prior to PRA treatment and compared to IFN-γ-primed ECs simply washed with PRA sera (t=0 control) (n=3, Student t-test, SEM). (D) IFN-γ-primed ECs were transfected with either control or gasdermin D siRNA and whole cell lysates were assessed for cleaved caspase-1 and culture supernatants were assessed for cleaved IL-1β. **P<0.01.
EC secreted IL-1 activates autocrine/paracrine induction of pro-inflammatory genes through canonical NF-κB signaling.
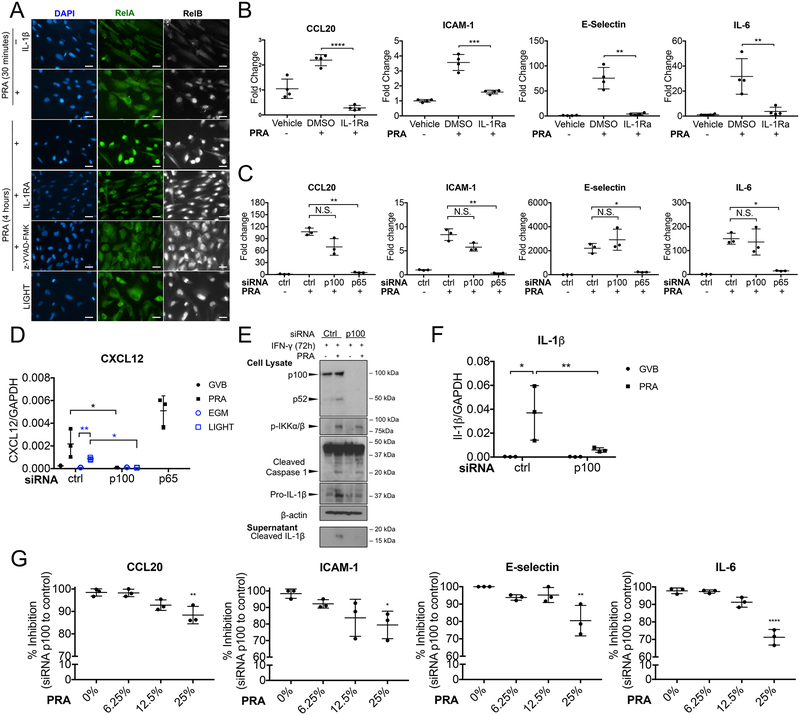
Our previous studies using siRNA revealed roles for NIK and non-canonical NF-κB components in the induction of pro-inflammatory genes such as CCL20, IL-6, E-selectin and ICAM-1 following deposition of MAC on ECs5. IL-1β is known to induce these same genes through canonical NF-κB activation and does not activate non-canonical NF-κB19, 20. Our new data revealing that MAC deposition on human ECs leads to IL-1β secretion posed the question: are these proteins being induced by an autocrine/paracrine pathway dependent on IL-1β and canonical NF-κB signaling? Consistent with our prior data, we observed nuclear translocation of RelB (indicative of non-canonical NF-κB signaling) but not of RelA nuclear translocation (indicative of canonical NF-κB signaling) at 30 minutes. However, RelA does translocate to the nucleus at later times (4 hours) (Figure 5A). Canonical but not non-canonical NF-κB signaling was blocked by pretreatment with either IL-1Ra or a caspase-1 inhibitor, indicating it is inflammasome- and IL-1-mediated (Figure 5A). Furthermore, the upregulation of transcripts encoding MAC-induced pro-inflammatory proteins was significantly abrogated by IL-1 receptor (IL-1R) blockade (Figure 5B) or by siRNA knockdown of RelA (Figure 5C). These data point to a critical role for autocrine/paracrine IL-1 activation of canonical NF-κB signaling in the induction of the pro-inflammatory phenotype of MAC-activated ECs. Treatment with LIGHT, which stabilizes NIK in the cytosol but fails to trigger assembly of an inflammasome, does not activate late phase canonical NF-κB signaling, suggesting that NIK stabilization is not sufficient to induce canonical signaling in the absence of inflammasome formation (Figure 5A). However, LIGHT is active in ECs as it induces RelB nuclear translocation (Figure 5A) and knockdown of p100 eliminates the induction of CXCL12, an endothelial non-canonical NF-κB target gene (Figure 5D)21. In ECs treated with IFN-γ for 72 hours, a timepoint when pro-IL-1β protein wanes, knockdown of p100 did not prevent inflammasome activation by MAC, but did inhibit release of active IL-1β into the culture medium (Figure 5E, Supplemental Figure VA and VB). More specifically, knockdown of p100 reduced the augmented level of IL-1β mRNA (Figure 5F) and pro-protein (Figure 5E) after PRA treatment. The extent of the inhibition of pro-inflammatory proteins depended on the strength of the MAC signal (Figure 5G). We interpret this to mean that the small amount of pro-IL-1β remaining after transient induction by IFN-γ may allow some IL-1β secretion, but it is much reduced compared to cells in which pro-IL-1β has been re-induced by NIK-mediated non-canonical NF-κB signaling (Figure 3I).
Figure 5. EC secreted IL-1 activates autocrine/paracrine induction of pro-inflammatory genes through canonical NF-κB signaling.
(A) IFN-γ-primed ECs were pre-treated with either DMSO, IL-1Ra, caspase-1 inhibitor z-YVAD-FMK prior to PRA sera treatment for 30 minutes or 4 hours, stained for either RelA or RelB and analyzed by epifluorescence. Scale bar, 25 μm. (B) ECs were pretreated with either DMSO or IL-1Ra (10ug/mL) to prior to PRA treatment for 8 hours or LIGHT treatment for 18 hours and transcript levels of pro-inflammatory genes CCL20, ICAM-1, E-selectin and IL-6 were assessed by qRT-PCR (n=4, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (C) qRT-PCR analysis of inflammatory gene expression by p65, p100 or control siRNA transfected IFN-γ-primed ECs after 25% PRA sera in GVB or vehicle treatment for 8 hours. (D) IFN-γ-primed ECs were transfected with control, p100 or p65 siRNA prior to PRA sera treatment for 8 hours and transcript of CXCL12 was analyzed by qRT-PCR (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (E) IFN-γ-primed ECs were transfected with p100 or control siRNA prior to PRA sera or vehicle treatment for 4 hours. Cell lysates were analyzed for cleaved caspase-1 and pro-IL-1β and culture supernatants were analyzed for cleaved IL-1β by immunoblot. (F) IFN-γ-primed ECs were transfected with control or p100 siRNA prior to PRA sera treatment for 8 hours and transcript of IL-1β was analyzed by qRT-PCR (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (G) Percent inhibition of inflammatory gene expression by p100 compared to control siRNA-transfected IFN-γ-primed ECs treated with varying levels of PRA sera in GVB for 8 hours. (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM) *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, n.s., non-significant.
NLRP3 inhibitor MCC950 blocks caspase-1 activation and pro-inflammatory gene induction in alloantibody and complement-treated ECs lining human coronary artery grafts in vivo.
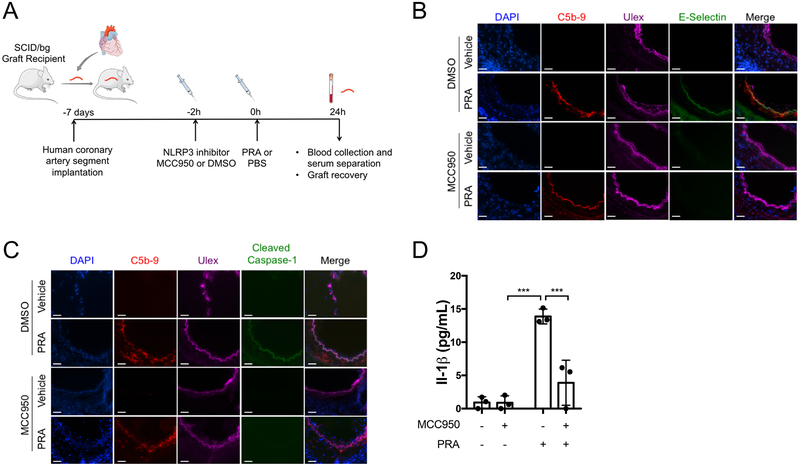
Although an enormous amount of EC biology has been discovered using cultured human umbilical vein ECs, cultured cells are different from ECs in situ. For this reason, we tested whether MAC-induced inflammasome activation occurred in human arterial ECs in vivo by implanting human coronary artery segments from a single donor into a set of four immunodeficient mice as aortic interposition grafts. Grafts were allowed to quiesce for 7 days at which time graft recipients were either pretreated with the NLRP3 inhibitor MCC950 or control DMSO in PBS prior to either PRA or control PBS injection (one mouse for each combination) without evidence of toxicity (Supplemental Figure VIA and VIB). The grafts were harvested 24 hours later and analyzed by immunofluorescence microscopy (Figure 6A). Complement deposition, cleaved caspase-1, and E-selectin, markers of complement activation, inflammasome assembly, and EC activation, respectively, were detected in the human ECs lining the artery grafts exposed to PRA sera but not PBS control (Figure 6B and 6C, top two rows). Complement activation but not cleaved caspase-1 or E-selectin staining in PRA-treated grafts pretreated with MCC950, consistent with the interpretation that PRA induced a NLRP3 inflammasome in graft ECs, and EC pro-inflammatory gene induction and caspase-1 activation were inhibited by MCC950 (Figure 6B and 6C, bottom two rows). Furthermore, human IL-1β, which is predominantly expressed by ECs lining the artery graft, was elevated in the serum of animals in the PRA only treatment group and was significantly attenuated by MCC950 pretreatment prior to PRA (Figure VID, Supplemental Figure VII). These results collectively suggest that the pathway observed in cell culture occurs in vivo and can be blocked by targeting the inflammasome in human ECs.
Figure 6. NLRP3 inhibitor MCC950 blocks caspase-1 activation and pro-inflammatory gene induction in alloantibody and complement-treated ECs lining human coronary artery grafts in vivo.
(A) Human coronary artery grafts from a single donor were implanted into a set of four immunodeficient mice and quiesced for 7 days prior to pre-treatment with NLRP3 inhibitor MCC950 or control DMSO in PBS. Following either PRA or PBS treatment, grafts were recovered and serum was collected after 24 hours. The experiment was repeated three times with different artery donors. For all treatment groups, n=3. (B) ECs lining arterial grafts were analyzed for complement C5b-9 and pro-inflammatory gene E-selectin staining by immunofluorescence. Scale bar, 50 μm. (C) ECs lining arterial grafts were analyzed for evidence of terminal complement C5b-9 staining and cleaved caspase-1 by immunofluorescence. Scale bar, 50 μm. (D) Serum was collected at time of graft recovery and assayed for human IL-1β by ELISA (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). ***P<0.001.
Caspase-1 inhibition or IL-1 receptor blockade reduce the capacity of MAC-activated human ECs to stimulate alloreactive CD4+ memory T cell responses.
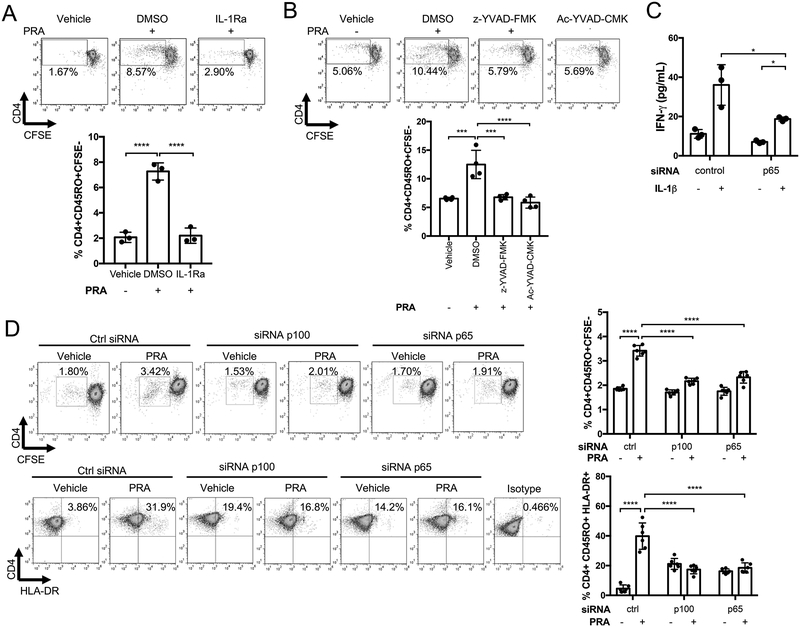
We previously reported that alloantibody and complement activation potentiated the ability of ECs to recruit and activate allogeneic T cells, likely in response to MAC-induced inflammatory gene expression5 that is actually mediated by IL-1β. We therefore assessed whether the enhanced EC-mediated T cell response induced by PRA treatment is also dependent on IL-1 signaling. IFN-γ-primed ECs were treated with IL-1Ra, incubated with PRA or vehicle for 6 hours, washed and then co-cultured with human allogeneic memory CD4+ T cells for 7 days. As before, PRA-treatment of ECs led to enhanced alloreactive memory T cell proliferation, as detected by CFSE dilution in EC:T cell cocultures, and the augmented response was significantly reduced by IL-1Ra treatment of ECs (Figure 7A). Treatment of IFN-γ-primed ECs with selective caspase-1 inhibitors, z-YVAD-FMK or Ac-YVAD-CMK prior to activation by PRA treatment also significantly reduced the enhanced levels of alloreactive memory CD4+ T cell proliferation (Figure 7B). To determine if active IL-1β is acting on ECs, T cells or both, we used siRNA to knock down p65 in ECs to inhibit their IL-1 responses and added exogenous IL-1β to EC:T cell cocultures. As effects of the siRNA are transient, we assessed T cell activation at 24 hours by analyzing the culture supernatants for IFN-γ instead of assessing proliferation at 7 days. We observed that siRNA knockdown of p65 largely attenuated but did not completely inhibit IL-1-augmented IFN-γ induction in the T cells (Figure 7C), suggesting that a significant part of the effects of MAC involve autocrine/paracrine actions of IL-1 on the ECs. These data do not preclude the possibility that IL-1 may have additional direct actions on the responding T cells. We next performed siRNA-mediated knockdown of p100 or p65 in ECs prior to PRA treatment and subsequent coculture with allogeneic CD4+ memory T cells. We observed that the enhanced activation of allogeneic T cells was significantly abrogated with knockdown of either p100 or p65 in ECs (Figure 7D). These data show that enhanced ability of PRA-treated ECs to recruit and activate memory CD4+ T cells is dependent on MAC-induced inflammasome assembly, pro-IL-1β synthesis by noncanonical NF-κB, and downstream IL-1 signaling through canonical NF-κB.
Figure 7. Caspase-1 inhibition or IL-1 receptor blockade reduce the capacity of MAC-activated human ECs to stimulate alloreactive CD4+ memory T cell responses.
(A) Proliferation of allogeneic CD4+ T cells after co-culture for 7 days with IFN-γ-primed-ECs pre-treated with IL-1Ra (10 μg/mL) or DMSO and PRA or vehicle treatment. CFSE dilution was assessed on day 7 (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (B) Memory CD4+ T cell proliferation after co-culture with IFN-γ-primed-ECs pre-treated with caspase-1 inhibitors Ac-YVAD-CMK (20μM) or z-YVAD-FMK (20μM) or DMSO prior to PRA sera or vehicle treatment for 6 hours. CFSE dilution was assessed on day 7 (n=4, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (C) IFN-γ production by allogeneic memory CD4+ T cells after co-culture with IFN-γ-primed-ECs transfected with control or p65 siRNA prior to addition of exogenous IL-1β or mock treatment. IFN-γ production was assessed by ELISA after 24 hours for coculture (72 hours after siRNA transfection). (n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM). (D) Proliferation and activation of CFSE-labelled memory CD4+ T cells after co-culture with IFN-γ-primed-ECs transfected with p100, p65 or control siRNA and PRA or vehicle treated for 6 hours. CFSE dilution was assessed on day 7. (n=6, one-way ANOVA and Tukey’s multiple comparisons test, SEM). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
DISCUSSION
The endothelium plays a major immunological role in tissue homeostasis, including host defense1. In organ transplantation, the immune system behaves as if it is defending against an infection. Allograft ECs are the principal cell type that express non-self HLA molecules (which in humans includes both class I and II) and are therefore the primary cellular targets detected by recipient humoral and cellular alloimmunity22. When graft ECs are targeted by donor-specific antibodies and complement activation, the ECs become activated and directly participate in pro-inflammatory responses through augmenting leukocyte adhesion, recruitment and activation and secretion of cytokines and chemokines4. Activated ECs become more immunogenic and augment recipient T cell-mediated responses. The research described here is part of our ongoing effort to uncover how complement-mediated signaling mechanisms alter the immunogenicity of ECs and allow for their enhanced capacity to modulate alloimmune responses. Key new findings of this study are that NIK, when stabilized on the surface of Rab5+ endosomes containing internalized MAC, recruits NLRP3 from the ER to Rab5+ endosomes, triggering inflammasome activation and IL-1β secretion. IL-1β then acts in an autocrine/paracrine manner to activate the ECs through canonical NF-κB signaling, augmenting the capacity of ECs to initiate alloimmune T cell responses. This process is primed by IFN-γ induction of NLRP3, pro-caspase 1 and gasdermin D. Endosomal NIK also activates non-canonical NF-κB signaling, which acts to increase synthesis of pro-IL-1β.
This pathway differs from previously described mechanisms of NLRP3 inflammasome assembly and further advances the concept that Rab5+MAC+NIK+ endosomes represent a signaling platform. While a diverse number of endogenous triggers such as ATP have been described to induce the NLRP3 inflammasome, we observed that MAC needs to be internalized from the cell surface and transferred to Rab5+ endosomes, allowing for accumulation of endosomal NIK, which was required for inflammasome assembly. In this way, the mechanism of MAC induction of the NLRP3 inflammasome differs from other pore-forming complexes and toxins, such as aerolysin, that have been shown to activate both NLRP3 and IPAF inflammasomes. Upon MAC deposition and internalization and unlike in response to ATP, the inflammasome sensor NLRP3 is selectively recruited from the ER, where it is known to localize in resting cells, to Rab5+ endosomes prior to oligomerization of ASC. Thus, Rab5+MAC+ endosomes not only serve as important signaling sites for NIK stabilization and activation of non-canonical NF-κB signaling, but also may be a nidus for the assembly of inflammasome components. Further analyses may reveal other Rab5+ endosomal signaling events in addition to non-canonical NF-κB activation and inflammasome assembly.
Activation of the NLRP3 inflammasome has been previously found to depend on a priming signal through PRRs and the MyD88 adaptor that upregulate NLRP3 and pro-IL-1β through canonical NF-κB activation. As cultured ECs lose HLA expression, our in vitro experiments routinely involve pre-treatment of the cultures with IFN-γ to re-induce these molecules. Our new results reveal that upregulation of inflammasome components and gasdermin D can be independent of MyD88 and instead dependent on activation of STAT1 by IFN-γ. IFN-γ treatment sensitized ECs to forming an NLRP3 inflammasome, as detected by caspase-1 activation and IL-1β release. Pro-IL-1β expression induction by IFN-γ was transient and therefore likely to be insufficient in vivo to support IL-1β synthesis because basal stimulation by IFN-γ is chronic. Instead, sustained induction of pro-IL-1β depends, at least initially, on non-canonical NF-κB signaling.
Our use of human materials allows us to gain insights that may not be possible using rodent models. For example, the association of DSA with chronic rejection in patients is most often associated with antibodies reactive with class II HLA molecules23. Humans but not mice and rats basally express class II molecules on their microvascular ECs8. Our findings that the inflammasome is activated in patient CAMR biopsies demonstrate the clinical relevance of this pathway. The inhibition of complement-mediated NLRP3-inflammasome activation and EC activation in human coronary artery graft ECs in vivo by NLRP3 inhibitor MCC950 reveal potentially new druggable molecular targets. Targeting an inflammasome inhibitor to the vasculature of the graft may be beneficial for transplant patient who develop de novo DSA.
There has been recent interest in the use of IL-1 inhibitors, including neutralizing antibodies and IL-1Ra, in ameliorating sequelae associated with inflammatory disorders, including pathologies associated with atherosclerosis24. Our results have clinical implications for supporting investigation with IL-1 blocking biologics in the setting of chronic rejection. Furthermore, complement effects on ECs are much broader than allotransplantation. Antibody binding and complement activation causing EC injury and activation occur in numerous settings outside of transplantation and the mechanism described in this report may have broader implications for immunopathology and treatment. For example, ischemia reperfusion injury (IRI) commonly occurs after restoration of blood flow to tissues or organs after a prolonged period of occlusion in clinical conditions such as stroke, myocardial infarction and post-transplantation. EC changes induced by IRI leads to binding of natural IgM or mannose binding lectin and leads to complement activation25, 26 and in experimental models of IRI, inhibition of complement reduces tissue injury27, 28. Complement activation by autoantibody or by immune complexes has also been implicated in systemic forms of autoimmunity such as systemic lupus erythematosus29 and rheumatoid arthritis30. In these settings, complement-mediated EC activation may contribute to the recruitment of pathogenic CD4+ T cells to the affected tissues30, 31. Presently, one complement inhibitor is clinically approved and many others are in development. As complement plays a variety of homeostatic roles in addition to its effector functions, broadly targeting complement may produce clinical implications32. Targeting a more specific response associated with inflammatory injury, such as the inflammasome or IL-1, may be more effective in controlling these pathologies.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is Known?
Solid organ transplantation is the only curative treatment for end-stage kidney, liver, lung and heart failure.
Chronic immune attack against organ graft vessels, commonly referred to as allograft vasculopathy, results in diffuse vascular stenoses, tissue malperfusion and graft loss.
Human anti-donor human leukocyte antigen (HLA) antibodies that activate complement and deposit complement membrane attack complexes (MACs) on human endothelial cells (ECs) initiate signaling events that alter human ECs to increase their capacity to activate alloreactive effector memory human T cells to proliferate and produce interferon-γ (IFN-γ).
What New Information Does This Article Contribute?
Internalized MACs stabilize NF-kB inducing kinase (NIK) to trigger assembly of an NLRP3 inflammasome in and interleukin-1β (IL-1β) secretion from IFN-γ-primed human ECs.
EC-derived IL-1β-mediates endothelial cell activation to augment the recruitment and activation of alloreactive T cells.
This study shows that MAC signaling initiates the assembly of NLRP3 inflammasome and subsequent IL-1β secretion from ECs.. Mechanistically, complement membrane attack complexes (MACs), deposited on the surface of human ECs in response to human alloantibody, are rapidly internalized into Rab5+ endosomes where they rapidly initiate signaling pathways by stabilizing NF-kB inducing kinase (NIK). NIK then activates two distinct responses: it initiates non-canonical NF-kB signaling which, among other things, induces transcription of pro-IL-1β and it initiates assembly of an NLRP3 inflammasome that processes and secretes mature IL-1β. IL-1β feeds back on the ECs to induce multiple other pro-inflammatory genes that increase the capacity of the ECs to activate alloreactive effector memory CD4+ T cells. Our work addressing complement-activated EC inflammasome assembly, could have broader implications for immune-mediated injury in multiple clinical settings.
ACKNOWLEDGMENTS
We thank Gwendolyn Davis-Arrington for HUVEC isolation and Verena Broecker for collection and diagnosis of human renal biopsies.
SOURCES OF FUNDING
NIH R01-HL051014 to J.S.P.; NIH R01-HL141137–01, R00-HL125895, and P30-AR053495–01A1 to D.J., and Vasculitis Foundation UL1TR001863 to D.J. NIH F30-AI138473-01A1 and NIH MSTP T32-GM007205 to C.B.X. Renal biopsies were performed at Addenbrooke’s Hospital, Cambridge, UK, for diagnostic purposes. Unused tissues were de-identified and used for research with ethical approval. Collection, diagnosis and tissue processing in Cambridge was supported by the NIHR-funded Cambridge Biomedical Research Centre.
Nonstandard Abbreviations and Acronyms:
- ATP
adenosine triphosphate
- CAMR
chronic antibody-mediated rejection
- DN
dominant-negative
- DSA
donor-specific antibodies
- ECs
endothelial cells
- GFP
green fluorescent protein
- GSDMD
gasdermin D
- HLA
human leukocyte antigen
- HUVECs
human umbilical vein endothelial cells
- IL-1Ra
IL-1 receptor antagonist
- MAC
membrane attack complexes
- NIK
NF-κB–inducing kinase
- PRA
panel reactive antibody
- PRRs
pattern recognition receptors
- WT
wildtype
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Abrahimi P, Liu R, Pober JS. Blood Vessels in Allotransplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15:1748–1754 [DOI] [PubMed] [Google Scholar]
- 2.Carman CV, Martinelli R. T Lymphocyte-Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity. Frontiers in immunology. 2015;6:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karahan GE, Claas FHJ, Heidt S. B Cell Immunity in Solid Organ Transplantation. Frontiers in immunology. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross AR, Glotz D, Mooney N. The Role of the Endothelium during Antibody-Mediated Rejection: From Victim to Accomplice. Frontiers in immunology. 2018;9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-kappaB signaling in endothelial cells. Circulation. 2013;128:2504–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jane-wit D, Surovtseva YV, Qin L, Li G, Liu R, Clark P, Manes TD, Wang C, Kashgarian M, Kirkiles-Smith NC, Tellides G, Pober JS. Complement membrane attack complexes activate noncanonical NF-kappaB by forming an Akt+ NIK+ signalosome on Rab5+ endosomes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9686–9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145 [DOI] [PubMed] [Google Scholar]
- 8.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends in immunology. 2012;33:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cernoch M, Viklicky O. Complement in Kidney Transplantation. Frontiers in medicine. 2017;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar CA, Sacks SH. Mechanisms of rejection: role of complement. Current opinion in organ transplantation. 2014;19:8–13 [DOI] [PubMed] [Google Scholar]
- 11.Latz E The inflammasomes: mechanisms of activation and function. Current opinion in immunology. 2010;22:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends in biochemical sciences. 2016;41:1012–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832 [DOI] [PubMed] [Google Scholar]
- 14.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225 [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature medicine. 2015;21:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665 [DOI] [PubMed] [Google Scholar]
- 18.Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity. 2018;48:35–44.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1995;9:899–909 [PubMed] [Google Scholar]
- 20.Denk A, Goebeler M, Schmid S, Berberich I, Ritz O, Lindemann D, Ludwig S, Wirth T. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. The Journal of biological chemistry. 2001;276:28451–28458 [DOI] [PubMed] [Google Scholar]
- 21.Madge LA, Kluger MS, Orange JS, May MJ. Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells. Journal of immunology (Baltimore, Md.: 1950). 2008;180:3467–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merola J, Jane-Wit DD, Pober JS. Recent advances in allograft vasculopathy. Current opinion in organ transplantation. 2017;22:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. The New England journal of medicine. 2013;369:1215–1226 [DOI] [PubMed] [Google Scholar]
- 24.Baylis RA, Gomez D, Mallat Z, Pasterkamp G, Owens GK. The CANTOS Trial: One Important Step for Clinical Cardiology but a Giant Leap for Vascular Biology. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:e174–e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. American journal of physiology. Heart and circulatory physiology. 2009;297:H1853–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766 [DOI] [PubMed] [Google Scholar]
- 27.Danobeitia JS, Ziemelis M, Ma X, Zitur LJ, Zens T, Chlebeck PJ, Van Amersfoort ES, Fernandez LA. Complement inhibition attenuates acute kidney injury after ischemia-reperfusion and limits progression to renal fibrosis in mice. PloS one. 2017;12:e0183701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin L, Li G, Kirkiles-Smith N, Clark P, Fang C, Wang Y, Yu ZX, Devore D, Tellides G, Pober JS, Jane-Wit D. Complement C5 Inhibition Reduces T Cell-Mediated Allograft Vasculopathy Caused by Both Alloantibody and Ischemia Reperfusion Injury in Humanized Mice. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16:2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Annals of the rheumatic diseases. 2014;73:1601–1606 [DOI] [PubMed] [Google Scholar]
- 30.Trouw LA, Pickering MC, Blom AM. The complement system as a potential therapeutic target in rheumatic disease. Nature Reviews Rheumatology. 2017;13:538. [DOI] [PubMed] [Google Scholar]
- 31.Mellado M, Martinez-Munoz L, Cascio G, Lucas P, Pablos JL, Rodriguez-Frade JM. T Cell Migration in Rheumatoid Arthritis. Frontiers in immunology. 2015;6:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaabak M, Babenko N, Shapiro R, Zokoyev A, Dymova O, Kim E. A prospective randomized, controlled trial of eculizumab to prevent ischemia-reperfusion injury in pediatric kidney transplantation. Pediatric transplantation. 2018;22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.