Abstract
Complex genetic mechanisms are thought to underlie many human diseases, yet experimental proof of this model has been elusive. Here, we show that a human cardiac anomaly can be caused by a combination of rare, inherited heterozygous mutations. Whole-exome sequencing of a nuclear family revealed that three offspring with childhood-onset cardiomyopathy had inherited three missense single nucleotide variants in the MKL2, MYH7 and NKX2–5 genes. The MYH7 and MKL2 variants were inherited from the affected-asymptomatic father and the rare NKX2–5 variant (minor allele frequency=0.0012) from the unaffected mother. We used CRISPR-Cas9 to generate mice encoding the orthologous variants and found that compound heterozygosity for all three variants recapitulated the human disease phenotype. Analysis of murine hearts and human induced pluripotent stem cell–derived cardiomyocytes provided histologic and molecular evidence for the NKX2–5 variant’s contribution as a genetic modifier.
One sentence summary:
A combination of three inherited heterozygous missense single nucleotide variants underlying familial heart disease.
Introduction
The genetic etiologies of complex phenotypes or diseases, such as type 2 diabetes, Parkinson’s disease and cardiovascular disease are not fully understood (1–4). High-throughput DNA sequencing is establishing the landscape of genetic variation in the absence of disease, enhancing identification of pathogenic variants involved in Mendelian disorders (5). However, the lack of experimental approaches to test involvement of multiple genetic variants and their epistatic relationships has hampered mechanistic dissection of complex phenotypes, especially those involving oligo- or polygenic inheritance and genetic modifiers (3, 6, 7). Definitive genetic causes of congenital heart disease (CHD), the most common congenital malformation, have been particularly elusive (8, 9). Rare inherited and de novo monogenic aberrations account for ~10% of cases, based on a recent exome sequencing study of CHD trios, while copy number variants have been identified in ~25% of cases (10, 11). Oligogenic inheritance and the involvement of genetic modifiers may contribute to CHD and cardiomyopathies; however, experimental confirmation of this model is lacking (12, 13).
Recent improvements in gene editing facilitated by CRISPR-Cas technology provide the opportunity to test hypotheses involving the potential for oligogenic inheritance of disease (14). In parallel, the establishment of human pluripotent stem cell models of differentiation has fostered the ability to study aberrant regulatory events that occur during embryonic development in a human cellular context (15). Here, we utilize these advances to dissect a complex familial case of heart disease and identify a rare missense NKX2–5 variant that acts as a modifier, on both phenotypic and molecular levels, in conjunction with novel missense variants in the transcription factor MKL2, and the sarcomeric protein MYH7.
Familial Left Ventricular Noncompaction
Upon presentation of a two-month-old infant with congestive heart failure requiring mechanical ventilation and inotropic support, echocardiography revealed severely depressed left ventricular (LV) function and deep LV trabeculations, characteristic of a type of cardiomyopathy known as left ventricular non-compaction (LVNC) (Fig. 1A, right). LVNC is thought to represent failure of cardiomyocyte maturation during embryonic development and accounts for almost 10% of all cardiomyopathies, though its incidence may be underestimated (16). This defect exhibits variability in presentation from neonates to adulthood, but appears to have a congenital etiology.
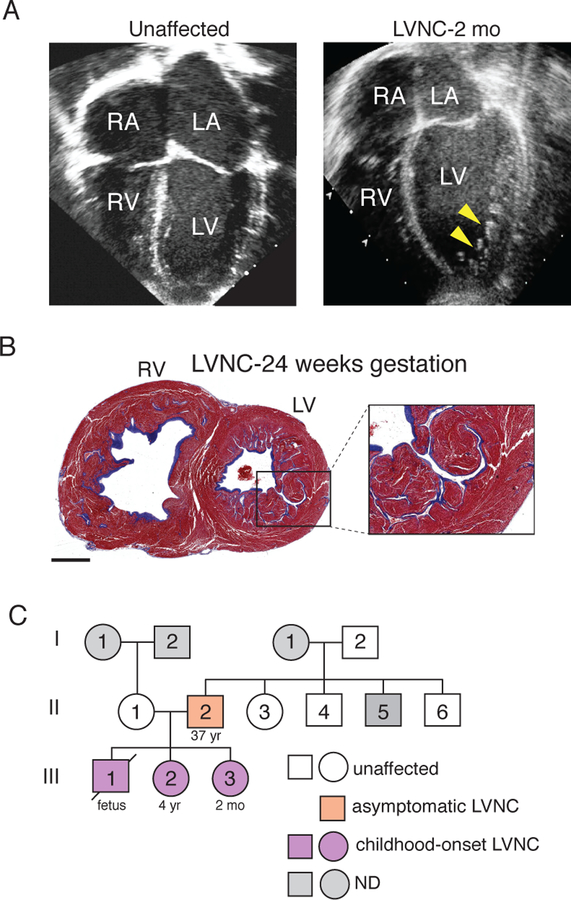
Fig. 1. Presentation of familial case of LVNC.
(A) Four-chamber echocardiography view showing the left atria (LA), right atria (RA), left ventricle (LV) and right ventricle (RV). Unaffected at left (43), index patient with LVNC at right. Yellow arrowheads indicate abnormal hypertrabeculation of the dilated LV. mo=month. (B) Transverse section of heart from sibling of index patient who had fetal demise. Higher magnification of the LV is at right. Scale bar = 6mm. (C) Pedigree showing inheritance pattern of LVNC in this family. Light grey indicates individuals whose cardiac status was not determined (ND) and white indicates unaffected individuals. Orange indicates the asymptomatic adult individual and purple indicates childhood-onset LVNC.
A family medical history disclosed a sibling that suffered fetal demise at 24 weeks of gestation. Initial autopsy results concluded that death was due to pulmonary hypoplasia. However, our examination of histologic sections revealed that the fetus suffered from biventricular noncompaction, based on the deep recesses in the myocardial walls of both ventricles, right ventricular dilation, and widespread fibrosis (Fig. 1B). Cardiac imaging of immediate living family members exposed previously undetected evidence of LVNC in a 4-year-old sibling and subtle signs of LVNC in the father (fig. S1A and B). The proband’s paternal grandfather had a history of arrhythmia but, similar to the extended family, no cardiac functional or structural abnormalities were detected (Fig. 1C). Collectively, these findings suggested vertical transmission of LVNC from the father with a markedly increased severity of disease and age of onset in offspring.
Multiple genetic variants segregate with familial left ventricular non-compaction
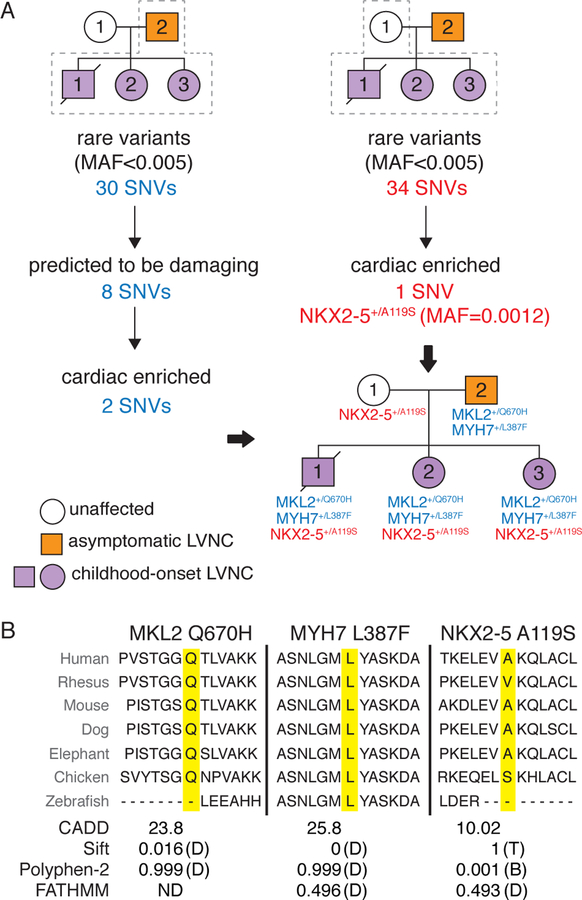
To investigate potential genetic causes of LVNC in this family, we performed whole-exome sequencing on the immediate family of the proband. We reasoned that individual de novo events were unlikely the cause of disease due to the penetrance in all three offspring, and instead pursued an inherited variant hypothesis. We initially focused on inherited private and/or rare (minor allele frequency, MAF<0.005) exonic non-synonymous variants inherited from the father and identified thirty single nucleotide variants (SNVs) of interest (Fig. 2A, left). Eight of these variants were predicted to be damaging, but only two were enriched in heart tissue transcripts (table S1).
Fig. 2. Genotypic analysis of LVNC family by exome sequencing.
(A) Workflow for analysis of sequence variants inherited from the father is shown in blue on the left and from the unaffected mother in red on the right. Inheritance pattern for variants of interest is shown in pedigree on lower right. (B) Conservation of each amino acid residue across species is shown. The variant of interest is highlighted in yellow. Predicted effects of variants on protein function based on Combined Annotation Dependent Depletion (CADD), Sift, Polyphen-2 and FATHMM programs. Damaging (D), benign (B), tolerated (T) and not determined (ND). Single nucleotide variant (SNV); Minor allele frequency (MAF).
The first variant of interest present only in the 4 family members with image-based evidence of LVNC was a novel heterozygous missense variant in myosin heavy chain 7 (MYH7) involving a leucine-to-phenylalanine substitution at position 387 (L387F) (Fig. 2A, left). This amino acid residue is highly conserved, predicted to be damaging by multiple algorithms, and resides within the ATPase domain of the protein (Fig. 2B, S2A). SNVs within this gene have previously been associated with hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM) and LVNC (16, 17). One interpretation of the phenotypic variability associated with variants in MYH7 is that genetic modifiers may be involved, although this has not been demonstrated (17, 18).
The second variant that met our filtering criteria in individuals with phenotypic evidence of LVNC resulted in a previously undescribed heterozygous glutamine-to-histidine substitution at position 670 (Q670H) in the transcription factor MKL2 (also known as myocardin-related transcription factor B, MRTF-B) (19). This residue is adjacent to the leucine zipper domain, is highly conserved and also predicted to be damaging by multiple algorithms (Fig. 2A and B, S2A). Myocardin activity at paired MEF2 sites requires leucine zipper domain–dependent homodimizeration (20). The myocardin family of regulators are potent transcriptional co-activators of SRF and Mef2c, both essential for cardiogenesis. Consistent with a detrimental effect of the variant, we found that MKL2 Q670H had reduced transcriptional activity in vitro compared to wild-type MKL2 (Fig. S2B). Homozygous Mkl2 deletion leads to defects in the cardiovascular epithelial-to-mesenchymal transition during mouse embryonic development and disrupts vascular development (21). While this gene has not previously been associated with cardiac disease, loss of function variants are underrepresented in the human population (o/e= 0.07), suggesting that the gene is important for human viability (22).
Given the marked increase in severity of disease in the three children compared to the father, we explored whether variants inherited from the unaffected mother might serve as genetic modifiers of the LVNC phenotype. We applied the same filtering criteria as described earlier, but in this case, we did not require variants of interest to be damaging based on traditional metrics (e.g. Sift or Polyphen) as we reasoned that genetic modifiers inherited from an unaffected parent could have a relatively subtle alteration of function. We identified 34 novel and/or rare variants (MAF <0.005) inherited by all three affected children from the unaffected mother (Fig. 2A, right). After filtering for those that were cardiac-enriched (23), we focused on a rare heterozygous missense variant in NKX2–5 that produced an alanine-to-serine substitution at position 119 (A119S, MAF=0.0012). NKX2–5 is an essential “core” transcriptional regulator of cardiac development and heterozygous mutations have been associated with CHD (24, 25). The alanine residue at position 119 is not conserved in Rhesus macaques, although the hydrophobicity at this site is maintained by the valine substitution in Rhesus (Fig. 2B). The NKX2–5 A119S variant is not predicted to be damaging (Fig. 2B) and is outside the critical DNA-binding homeodomain (fig. S2A), but it has been reported to slightly reduce DNA binding in vitro and has been noted in the setting of CHD and cardiomyopathies, although with incomplete penetrance (26–28).
Sequence analysis validated all three variants (fig. S2C, table S2) and sequencing of all paternal family members exposed the presence of the MKL2 Q670H variant in an unaffected uncle of the proband and a grandfather, both phenotyped by echocardiography, indicating that this variant is not sufficient to cause cardiac dysfunction (fig. S2D). The SNV in MYH7 arose de novo in the father (fig. S2D). The observation of isolated MKL2 Q670H or NKX2–5 A119S variants in unaffected individuals raised the possibility that these alterations have subtle effects on protein function and either have no consequence or may act as modifiers of the phenotype in the presence of the MYH7 L387F variant. While it is possible that other genetic variants and environmental factors may contribute to the severity of the disease, the inheritance pattern of the heterozygous missense variants in MKL2, MYH7 and NKX2–5 led us to hypothesize that their collective inheritance was sufficient to cause LVNC.
Functional significance of MKL2, MYH7 and NKX2–5 missense variants in vivo
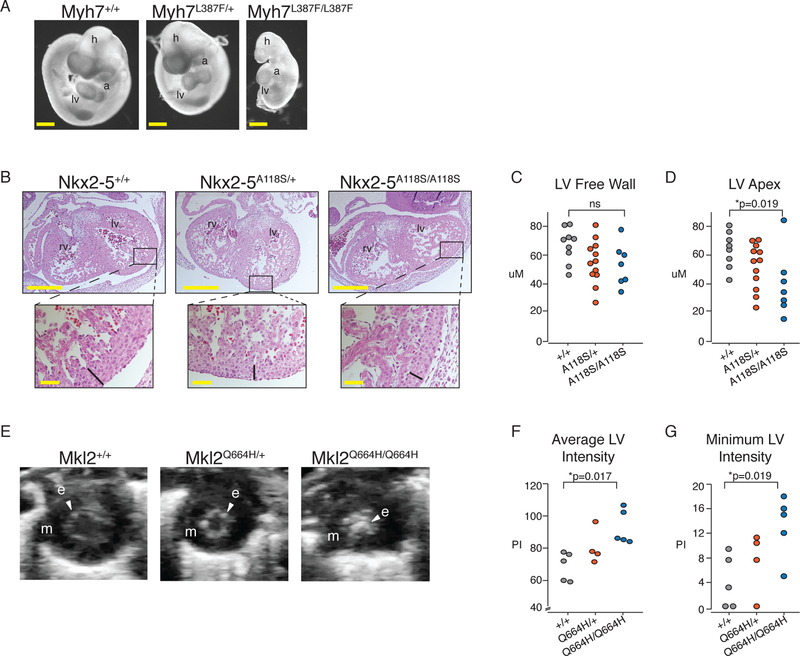
To evaluate the in vivo functional consequence of the MKL2, MYH7 and NKX2–5 SNVs identified in this family, we generated mice (C57BL/6J) harboring the orthologous missense variants by CRISPR-Cas9 gene-editing (fig. S3A–C). Animals were bred to homozygosity to test the effects of each individual missense variant in vivo. While Myh7+/L387F animals were observed at the expected Mendelian ratio, homozygous animals died by embryonic day (E) 9.5–10.0 with evidence of heart failure (Fig. 3A). Thus, the MYH7 L387F substitution is damaging.
Fig. 3. Functional Evaluation of Disease-Associated SNVs in Mice.
(A) Left lateral images of E9.5 embryos from a representative Myh7L387F/+ x Myh7L387F/+ cross. h, head; lv, left ventricle; a, atrium. Scale bar = 5mm. (B) H&E stained transverse histologic sections of E13.5 embryos with indicated NKX2–5 genotypes. Width of compact layer indicated by black bar in higher magnification views of boxed areas. Top scale=500μM. Bottom scale=50μM. (C) Quantification (μM) of LV free wall thickness and (D) apical wall thickness from E13.5 mice collected from three litters of mice. P-value was calculated using a t-test. (E) Short axis view of LV in systole viewed by echocardiography of P4 mice with indicated Mkl2 genotypes. m, myocardium signified by dark area; e, endocardium indicated by arrowheads. (F) Average pixel intensity (PI) and (G) minimum PI within the LV of P4 mouse hearts calculated from three litters of mice. P-value was calculated using a t-test.
Mendelian ratios were observed for NKX2–5+/A118S or Mkl2+/Q664H mice, and although animals homozygous for each did not exhibit evidence of cardiac dysfunction by echocardiography, they had subtle abnormalities in the ventricular wall before the first week of life. At embryonic time points, we noted a thin apical myocardial wall in NKX2–5A118S homozygous mice (Fig. 3B–D). In Mkl2Q664H homozygous mice, marked echogenic foci in the left ventricular cavity of postnatal day 4 (P4) homozygous mice were present, similar to that in human patients with LVNC (Fig. 3E–G, Movies S1–S3). Previous work did not observe a similar phenotype in mice with cardiomyocyte-specific deletion of Mkl2, suggesting the echogenicity results from Mkl2’s involvement in the development of another cell type within the heart (29). These results indicate that the SNVs identified in MYH7, MKL2 and NKX2–5 adversely affect the ability of the encoded protein to promote timely ventricular development in a homozygous mouse model.
Triple compound heterozygous mice exhibit LVNC
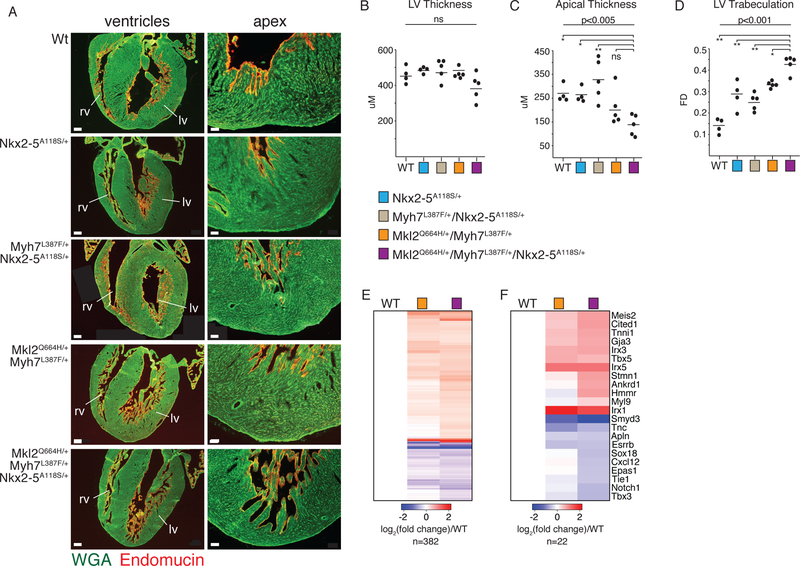
We next investigated whether compound heterozygosity of the MKL2, MYH7 and NKX2–5 variants produced a LVNC-like phenotype in mice. Immunohistochemistry with an antibody to the endocardial marker endomucin at P3 revealed mild hypertrabeculation and apical recesses in Mkl2Q664H/+Myh7L387F/+, and in NKX2–5A118S/+ mice (Fig. 4A). In contrast, triple heterozygote mice (Mkl2Q664H/+Myh7L387F/+NKX2–5A118S/+) exhibited deep trabeculations in the left ventricular wall similar to those seen in patients with LVNC and those observed in the autopsy of the affected child in the family described here (Fig. 4A). Quantification of P3 sections confirmed a decrease in the apical wall thickness and a statistically significant difference in trabecular complexity, but revealed no changes in LV free wall thickness (Fig. 4B–D). Few differences were noted between Myh7L387F/+NKX2–5A118S/+ and NKX2–5A118S/+ mice, illustrating the contribution of Mkl2Q664H/+ to the LV phenotype (Fig. 4A–D). There were no major phenotypic differences between WT and Myh7L387F/+ mice, consistent with the hypothesis that this variant does not independently lead to severe disease (fig. S4A).
Fig. 4. Mkl2Q664H/+Myh7L387F/+NKX2–5A118S/+ Compound Heterozygous Mice Recapitulate Features of Human LVNC.
(A) Left: representative histologic sections in four-chambered views of P3 mice. Scale bar = 200 mM. Right: higher magnification of the apical region of the same hearts shown on left. Scale bar = 50 mM. Immunohistochemistry with green indicating wheat germ agglutinin (cell membrane) and red marking endocardium (endomucin). (B) Quantification of LV free wall thickness, (C) apical wall thickness and (D) Trabeculation based on fractal dimension analysis. * P< 0.05, ** P< 0.01, ns=not significant. P-values calculated with one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Mean indicated by horizontal bar. Data collected from ten litters of mice. (E) Heatmap of all genes differentially expressed between WT and Mkl2Q664H/+Myh7L387F/+ or Mkl2Q664H/+Myh7L387F/+NKX2–5A118S/+ mice. Key at bottom indicates log2 fold change. (F) Heatmap of selected genes chosen from E. Heat map generated as in E.
Despite the ventricular hypertrabeculation, cardiac dysfunction by echocardiography was normal at baseline in adult Mkl2Q664H/+Myh7L387F/+NKX2–5A118S/+ mice (Fig. S3B and C). Transverse aortic constriction (TAC) was used to increase the pressure load on the left ventricle and caused a statistically significant reduction in cardiac function in triple heterozygous compared to wild-type mice (fig. S4D and E). This pathological response exhibited incomplete penetrance, suggesting that the triple heterozygous mice were on the threshold of functional abnormality.
RNA sequencing of tissue from the apex of P7 hearts revealed subtle yet statistically significant upregulation of genes associated with metabolism in triple compound heterozygous mice, whereas genes associated with vasculogenesis were downregulated (fig. S4F and G, table S3). Of 378 protein-coding genes differentially expressed between WT and triple heterozygous mice, 158 were also differentially expressed between WT and Mkl2Q664H/+Myh7L387F/+ mice, more than expected by chance (Fig. 4E, table S4) (Fisher’s test p-value =8.8e-155, odds ratio=37.6). However, 58% (224/378) of genes were uniquely differentially expressed in triple heterozygous mice, including many essential for cardiovascular development and function, highlighting the contribution of the NKX2–5 A118S variant.
Increased expression of genes involved in the cell cycle and mitosis was observed in triple heterozygous mice, supporting previous evidence that the non-compaction phenotype is associated with dysregulation of proliferation (fig. S4G)(30, 31). Genes expressed at higher levels in the myocardial trabeculae during embryonic development were also upregulated in triple mutant mice, supporting the histological observation of hypertrabeculation (Fig. 4F, table S4)(32). Genes associated with earlier stages of development and bound by NKX2–5 were additionally expressed at higher levels in triple compound heterozygous mice, consistent with a less mature state compared to wild-type (Fig. 4F)(33–36). Conversely, genes associated with endothelial cell development and the coronary vasculature were downregulated (Fig. 4F, table S4). Disruption of Notch signaling contributes to improper endothelial cell development and subsequent LVNC through reciprocal interactions with the myocardium, suggesting poor endothelial cell function may be associated with the LVNC phenotype (30, 37). Consistent with our observations, RNA sequencing after cardiomyocyte-specific deletion of Mkl1/2 hearts similarly demonstrated dysregulation of epithelial cell–related pathways, but no disruption in cardiomyocyte function (29). While we cannot exclude the possibility that additional variants influence the disease phenotype in humans, these results suggest inheritance of the SNVs in Mkl2, Myh7 and NKX2–5 is sufficient to mimic the pathology of LVNC in a mouse model.
Human iPSC-derived cardiomyocytes exhibit disease-related alterations
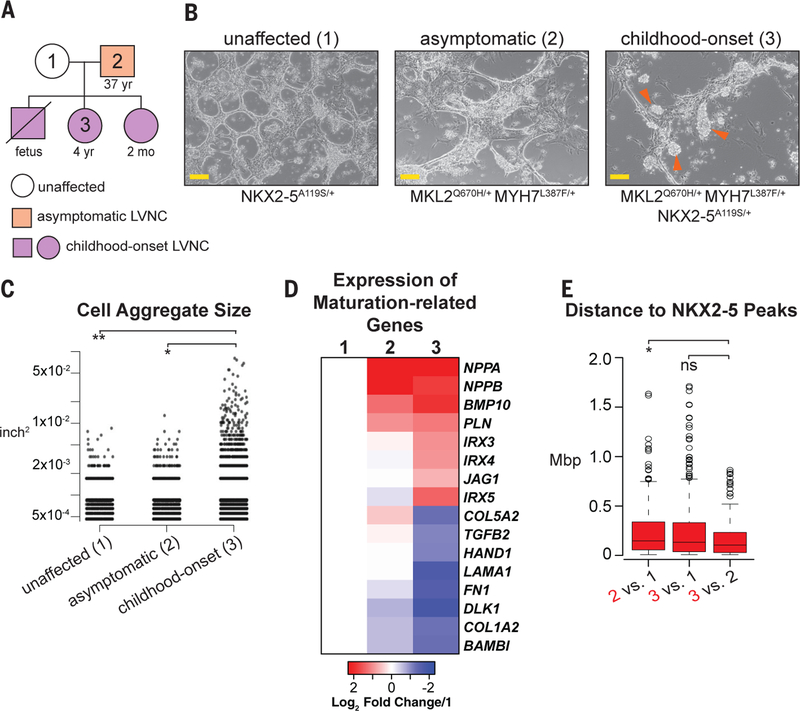
To determine if the phenotype exhibited by triple heterozygous mice reflected the effects of the genetic mutations in human cardiomyocytes, we generated patient-specific induced pluripotent stem cell (hiPSC) lines from multiple family members and differentiated them to cardiomyocytes via WNT pathway modulation (Fig. 5A and B) (38). Differentiation efficiency was similar in all lines, as assessed by cardiac troponin T expression (fig. S5A). However, discrepancies in adherence between patient lines became apparent by day 7 of differentiation as cells derived from an individual with symptomatic LVNC formed aggregates that were less apparent in the unaffected individual (Fig. 5B and C), consistent with previous work showing that Mkl2 regulates genes associated with cell adhesion (21).
Fig. 5. Human iPSC-derived Cardiomyocytes Exhibit features of LVNC.
(A) Pedigree of family members included in hiPSC-studies. (B) Brightfield images of hiPSC-derived cardiomyocytes from three family members as indicated. Scale = 100 mM. Orange arrowheads indicate abnormal aggregation. (C) Quantification of cellular aggregation. Y axis is log scale. n=2. (D) Heatmap depicting log2 fold change of cardiomyocyte maturation-related genes in asymptomatic (2) and symptomatic (3) LVNC hiPSC-derived cardiomyocytes, compared to unaffected (1). Key at bottom. Full list can be found in Table S6. (E) Box plot illustrating the distance from genes expressed at high levels in the individual labeled in red to the closest NKX2–5 ChIP-seq peak. Peak distances > 2 megabase pairs (Mbp) were excluded from the graph. P-values calculated using Wilcoxon rank-sum test with Bonferroni correction.
RNA-seq on day 8 of cardiomyocyte differentiation revealed downregulation of gene sets associated with cell adhesion and extracellular matrix deposition in the symptomatic case (MKL2Q670H/+MYH7L387F/+NKX2–5A119S/+), supporting the visual observation of decreased adhesion of these cells (fig. S5B). Gene ontology (GO) analysis revealed upregulation of cell cycle and cardiac developmental genes in cells derived from the symptomatic LVNC case, similar to that observed in triple heterozygous mice (fig. S5B, table S5). Evaluation of genes associated with the cardiac progenitor state and trabecular myocardium exhibited higher expression in the LVNC lines compared to the unaffected line (Fig. 5D, table S6). There was no temporal change in T (Brachyury) induction and repression, suggesting normal mesendoderm specification (fig. S5C); however, BMP10 exhibited delayed activation (fig. S5D).
A statistically significant overlap was observed between differentially expressed genes shared by the asymptomatic and symptomatic individual’s cell lines compared to unaffected (fig. S5E). However, in agreement with the mouse transcriptome data, the fold change of many key genes was often greater in the cell line derived from the individual diagnosed with symptomatic LVNC and harboring all three genetic variants (Fig. 5D). Despite hiPSC-derived cardiomyocytes representing an earlier stage of development than the mouse heart tissue included in this study (39), we also found that 43 genes were differentially expressed in both model systems (table S7).
To infer gene dysregulation that may be related to disruption of NKX2–5 function due to the A119S SNV, we utilized published NKX2–5 ChIP-sequencing data collected on day 10 of cardiomyocyte differentiation (table S8)(40). We found that genes expressed at higher levels in the triple heterozygous childhood-onset individual (Fig. 5A, #3) compared to her father (Fig. 5A, #2) are significantly closer to NKX2–5 binding events compared to gene sets identified in alternative differential expression scenarios and randomly permuted data (Fig. 5E; 3 vs. 2 to permuted peak set, p=7.12×10−13; 3 vs. 2 to randomly sampled expressed genes, p=0.002). The lack of association between genes expressed at lower levels may suggest that the populations from the unaffected and asymptomatic individuals have progressed to a developmental state whose gene signature is not associated with NKX2–5 binding (fig. S5G). Collectively, these results suggest that, while disruption of proper endothelial cell development and function may contribute to LVNC, there is a cardiomyocyte cell-autonomous component.
Discussion
The development of assays to test variants of unknown significance is essential for the advancement of precision medicine initiatives. While deletions, insertions and frameshift variants have predictable consequences, the effect of millions of SNVs identified in each individual’s genome are difficult to assess computationally. Recent advances in gene editing technologies have created an avenue to interrogate the contribution of these variants to phenotypes and disease (14). Our data suggest traditional metrics that identify phenotype-associated variants are likely not designed to identify the subtle effects of genetic modifiers (41)and that such genetic modifiers may explain the wide spectrum of cardiomyopathies observed among individuals with mutations in the same sarcomeric gene (42). Accordingly, additional experimentation and analysis will ultimately expose the prevalence and context in which NKX2–5 A119S, or other missense variants, can function as a modifier of CHD or cardiomyopathies.
Using human genetic variation to experimentally dissect biological processes will undoubtedly reveal novel insights regarding disease mechanisms. As we refine our understanding of the regulatory mechanisms that govern cell-autonomous and non-cell-autonomous cellular states using advances in both gene editing and single cell next-generation sequencing approaches, our ability to correlate genetic variation with phenotypic outcome will improve, bringing precision medicine closer to reality. While various genetic mechanisms, such as non-coding variation and omigenics, have advanced our understanding of the genetics underlying complex phenotypes, the work presented here suggests experimental exploration of SNV-associated phenotypes are a worthwhile endeavor and can shed light on the mechanisms of complex diseases.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Srivastava lab and members of Gladstone Institutes for helpful discussion and feedback; the Gladstone Histology and Microscopy, Stem Cell, Transgenic, and Genomics Core for making this work possible; the family who generously offered to be part of this study; and B. Taylor and K. Claiborn for editorial assistance.
Funding. C.A.G. is a HHMI fellow of the Damon Runyon Cancer Research Foundation (DRG-2206–14). Y.K.B. was a Gladstone CIRM scholar (TG2–01160). D.S. is supported by NHLBI/NIH grants (R01 HL057181, U01 HL098179, U01 HL100406) the Roddenberry Foundation, the L.K. Whittier Foundation and the Younger Family Fund. This work was also supported by NIH/NCRR grant C06 RR018928 to the Gladstone Institutes.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Competing Interests. D.S. is a co-founder of Tenaya Therapeutics.
Data and Materials availability. All raw data are available in the manuscript or deposited to GEO (GSE111394) and SRA (PRJNA531964).
REFERENCES AND NOTES
- 1.Kilpinen H, Dermitzakis ET, Hum. Mol. Genet 21, R24–R28 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Taylor MB, Ehrenreich IM, Trends Genet 31, 34–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle EA, Li YI, Pritchard JK, Cell 169, 1177–1186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera AV et al. , Nat. Genet 50, 1219–1224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lek M et al. , Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolio TA et al. , Nature 461, 747–753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riordan JD, Nadeau JH, Am. J. Hum. Genet 101, 177–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi S, Brueckner M, Circ. Res 120, 923–940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Linde D et al. , J. Am. Coll. Cardiol 58, 2241–2247 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Jin SC et al. , Nat. Genet 49, 1593–1601 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glessner JT et al. , Circ. Res 115, 884–896 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelb BD, Chung WK, Cold Spring Harbor Perspectives in Medicine 4 (2014), pp. a013953–a013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X et al. , Nat. Genet 49, 1152–1159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg SH, Doudna JA, Mol. Cell 58, 568–574 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Rowe RG, Daley GQ, Nat. Rev. Genet (2019), 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed]
- 16.Finsterer J, Stöllberger C, Towbin JA, Nat. Rev. Cardiol 14, 224–237 (2017). [DOI] [PubMed] [Google Scholar]
- 17.McNally EM, Barefield DY, Puckelwartz MJ, Cell Metab 21, 174–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J et al. , Sci. Rep 8 (2018), 10.1038/s41598-018-19372-4. [DOI] [Google Scholar]
- 19.Oh J, Richardson JA, Olson EN, Proc. Natl. Acad. Sci. U. S. A 102, 15122–15127 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CM et al. , Development 144, 1235–1241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trembley MA, Velasquez LS, de Mesy Bentley KL, Small EM, Development 142, 21–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karczewski KJ et al. , bioRxiv (2019), p. 531210.
- 23.Consortium GTEx et al. , Nature 550, 204–213 (2017).29022597 [Google Scholar]
- 24.Schott JJ et al. , Science 281, 108–111 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Lyons I et al. , Genes Dev 9, 1654–1666 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Reamon-Buettner SM et al. , PLoS One 8, e83295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dentice M et al. , J. Clin. Endocrinol. Metab 91, 1428–1433 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Costa MW et al. , Circ. Cardiovasc. Genet 6, 238–247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokalled MH et al. , Dev. Biol 406, 109–116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Amato G et al. , Nat. Cell Biol 18, 7–20 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luxán G et al. , Nat. Med 19, 193–201 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Christoffels VM, Keijser AG, Houweling AC, Clout DE, Moorman AF, Dev. Biol 224, 263–274 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Brody MJ et al. , J. Mol. Cell. Cardiol 62, 237–246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He A, Kong SW, Ma Q, Pu WT, Proc. Natl. Acad. Sci. U. S. A 108, 5632–5637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arber S, Halder G, Caroni P, Cell 79, 221–231 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Kuo H et al. , Development 126, 4223–4234 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Rhee S et al. , Nat. Commun 9, 368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burridge PW et al. , Nat. Methods 11, 855–860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLaughter DM et al. , Dev. Cell 39, 480–490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson DJ et al. , Nat. Commun 9 (2018), 10.1038/s41467-018-03714-x. [DOI] [Google Scholar]
- 41.Sun S et al. , Genome Res 26, 670–680 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starita LM et al. , Am. J. Hum. Genet 101, 315–325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madriago E, Silberbach M, Pediatr. Rev 31, 4–12 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.