Fig. 5. IL-1β-induced phosphorylation of RelB on S472 diminishes interaction with IκBα.
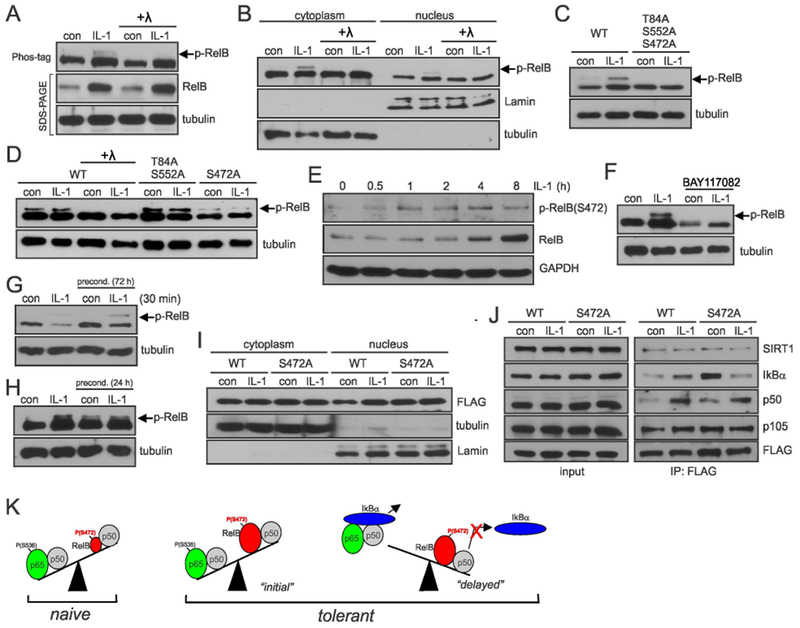
(A, B) Phosphorylation of RelB in astrocytes analyzed by Phos-Tag. Astrocytes were stimulated for 8h with IL-1β. Lysates were treated with lambda (λ) phosphatase as indicated. (C, D) Phosphorylation of RelB mutants in transfected HEK293 cells analyzed by Phos-tag. Cells were stimulated with IL-1β for 8h. (E) Phosphorylation of RelB in astrocytes was analyzed using phospho-RelB(S472) antibodies (F, G, H). Astrocytes were stimulated with IL-1β and/or BAY11-7082 and phosphorylation of RelB was analyzed by phos-tag. (I) Accumulation of overexpressed RelB in HEK293 cells analyzed by western blotting. (J) Analysis of interaction of WT-RelB and S472A mutant with binding partners in transfected HEK293 cells. (A-J) Representative blots of two-five experiments yielding similar data are shown. (K) Proposed model of tolerance: low levels of RelB ensure efficient cytokine induction by p65/p50 in naïve cells; in tolerant cells during the “initial” phase cytokine genes are activated by p65/p50 even though the presence of higher levels of RelB/p50; tolerance is established once IκBα is resynthesized and efficiently removes p65/p50 from the DNA. Phosphorylation of RelB at S472 prevents efficient removal of RelB/p50 complexes from the DNA making it inaccessible for p65/p50 to bind. Representative blots of 2-5 experiments are shown.
