Figure 4.
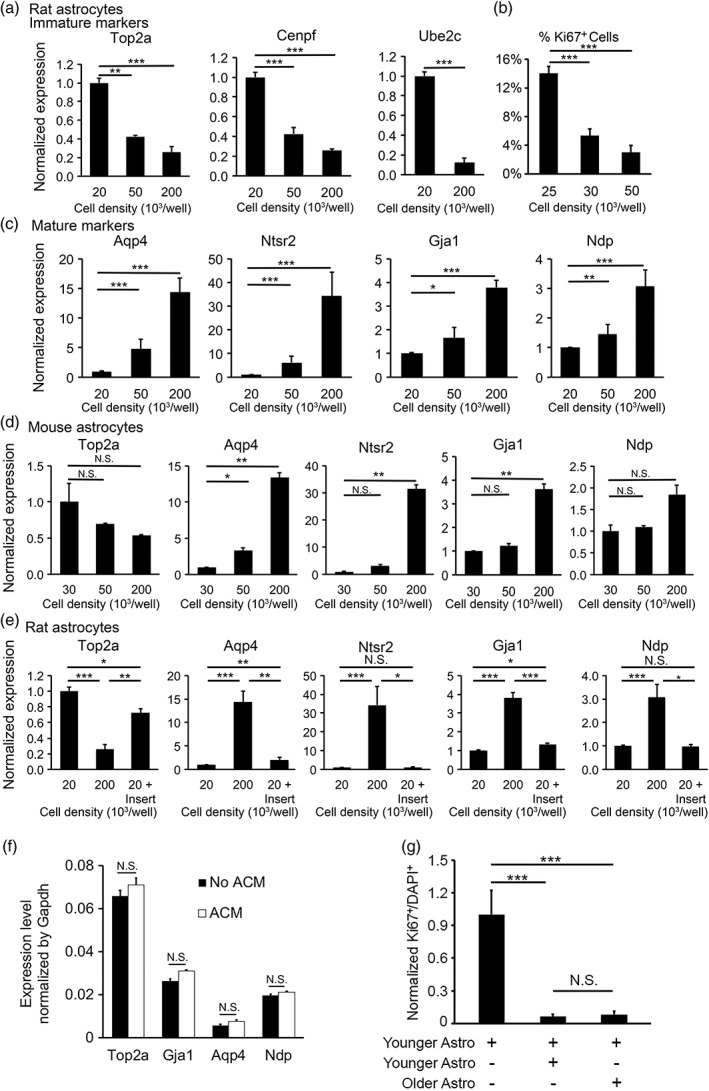
Astrocyte‐to‐astrocyte contact promotes astrocyte maturation. (a–c) Rat astrocytes matured faster at high cell density. We determined the expression of immature (a) and mature (c) astrocyte markers by qRT‐PCR. The expression for each gene was normalized to the house‐keeping gene Gapdh. The expression at each cell density was normalized to the level at 20 × 103 cells per well. (b) We determined the percentage of proliferating cells by quantifying Ki67 positive cells at 14 div. (d) Similar to rat astrocytes, mouse astrocytes matured faster at high cell densities. (e) We grew rat astrocytes at three conditions: Low density (20 × 103 cells per well), high density (200 × 103 cells per well), and low density with inserts (20 × 103 cells per well with additional 180 × 103 cells per well on porous inserts). Secreted signals freely diffused across the porous inserts whereas cells separated by the inserts do not make direct contacts. (f) Expression of astrocyte maturation markers as determined by qPCR in the presence and absence of 75 μg/mL ACM. (g) Younger and older astrocytes similarly promote astrocyte maturation. Younger astrocytes alone: Astrocytes purified from P2 rats plated at low density (30,000 per well). Younger + younger astrocytes: Astrocytes purified from P2 rats plated at high density (100,000 per well). Younger + older astrocytes: We first purified astrocytes from P7 rats and cultured them for 7 days before adding younger astrocytes purified from P2 rats. We labeled older astrocytes with lentiviruses encoding EGFP at >98% efficiency. We plated cells at varying densities and only used wells with the same final density in the latter two conditions for the final analyses. We quantified the percentage of proliferating cells by counting Ki67+ cells out of all DAPI stained cells. In the heterochronic condition, we only counted younger astrocytes (not labeled by EGFP). Percentage of Ki67+ cells was normalized to the younger astrocyte alone condition
