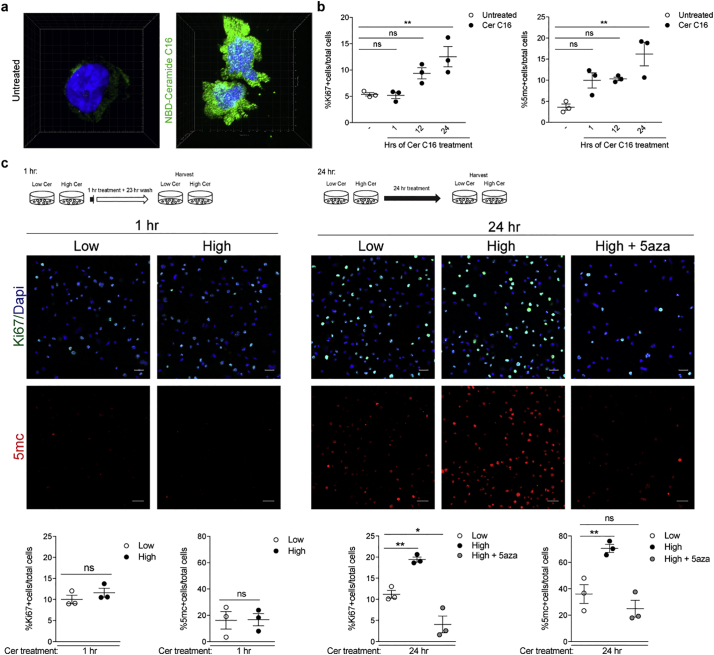
Fig. 3.
Exposure to ceramides results in dose-dependent increase in proliferation and DNA methylation. (a) Cultured monocytes (i.e. THP1 cells) were treated with fluorescently labeled Ceramide C16 (NBD-Ceramide C16) or untreated for 24 h. 3D reconstruction of the cells was conducted on Imaris to visualize localization of NBD-Ceramide (green). (b) Cultures either untreated or treated with Ceramide C16 (Cer C16) for 1, 12, or 24 h were fixed at the same time. After immunocytochemistry for Ki67 to detect proliferating cells or5-methylcytosine as marker for global DNA methylation the percentage of cells relative to the total DAPI+ nuclei was calculated and plotted in the graphs. Statistical differences in the percentage of Ki67+ cells and percentage of 5mc + cells relative to total DAPI+ nuclei were assessed by Student's t-test. (c) Cultures were treated for 1 (left panels) or 24 (right panels) hours with concentrations of a mixture of ceramides C16, C22, and C24:1, mimicking those detected in the plasma of MS patients with normal (Low) or high BMI (High) in the absence or presence of the DNA methylation inhibitor, 5-aza-2′-deoxycytidine (5-aza). After fixation, cells were stained for Ki67 (green) as proliferation marker, 5-methylcytosine (5mC, red), as marker for global DNA methylation and DAPI (blue), to identify nuclei. Confocal images were acquired at 20× magnification using the Zeiss LSM800 confocal (scale bar = 25um). High and High+5-aza groups were compared to Low Cer at 24 h using Dunnett's multiple comparisons test (*p < .05, **p < .01) (n = 3 independent biological replicates, each conducted in triplicate wells and analyzing 3 images/well). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)