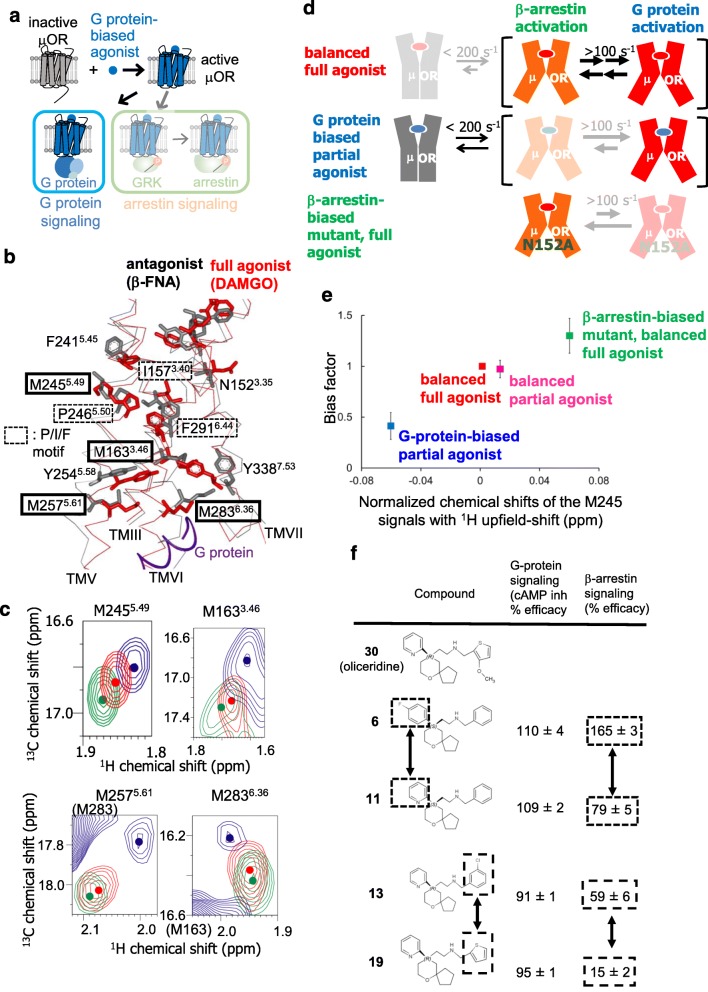
Fig. 2.
Biased signaling–related conformational dynamics of μ-opioid receptor. a Schematic diagram of signaling mediated by μOR in the presence of the G protein–biased agonist. b The crystal structures of μOR with an irreversible antagonist, β-funaltrexamine (PDB code: 4DKL) (black), and with a full agonist, BU72, and a G protein (PDB code: 6GDG) (red) are shown in side views with the extracellular sides on the upper sides, viewed from TMVI. The middle regions of TMIII, TMV, TMVI, and TMVII are shown in Cα traces, and the side-chains of N1523.35, I1573.40, M1633.46, F2415.45, M2455.49, P2465.50, M2575.61, M2836.36, F2916.44, and Y3387.53, and the bound ligands are depicted by sticks. The C-terminal region of the α subunit of the G protein is shown as a red ribbon. c Overlaid 1H-13C HMQC spectra of the [2H-8AA, αβ-2H-, methyl-13C-Met] μOR in the DAMGO-bound (red) and oliceridine-bound (blue) states and that of the μOR/N1523.35A mutant in the DAMGO-bound state (green). Only the regions with M2455.49, M2575.61, M2836.36, and the 1H upfield-shifted M1633.46 resonances are shown. The centers of the resonances from M1633.46, M2455.49, M2575.61, and M2836.36 are indicated with dots. d Mechanisms leading to the functional selectivity of μOR for different ligands. In the balanced full agonist state with DAMGO bound, μOR primarily adopts the active conformation (red), in which the intracellular surface is characterized by multiple substates with exchange rates larger than 100 s−1. In the G protein–biased partial agonist TRV130–bound state, MOR exists in an equilibrium between the inactive (gray) and active conformations, with exchange rates smaller than 200 s−1, and the equilibrium within the active substates is shifted toward the conformation preferred for G protein activation. In the DAMGO-bound state of the MOR N1523.35A mutant, where MOR adopts only the active conformation, the equilibrium among the active substates is shifted toward the conformation preferred for arrestin activation. e Correlation between the normalized chemical shifts of the M2455.49 NMR signals and the value of the bias factor, which is the ratio of the β-arrestin signaling efficacy to the G protein signaling efficacy. f Structure-function relationships of derivatives of the MOR ligand, oliceridine. Modification of either of the two aromatic rings that are highlighted in boxes results in the selective modulation of β-arrestin signaling