Fig. 1.
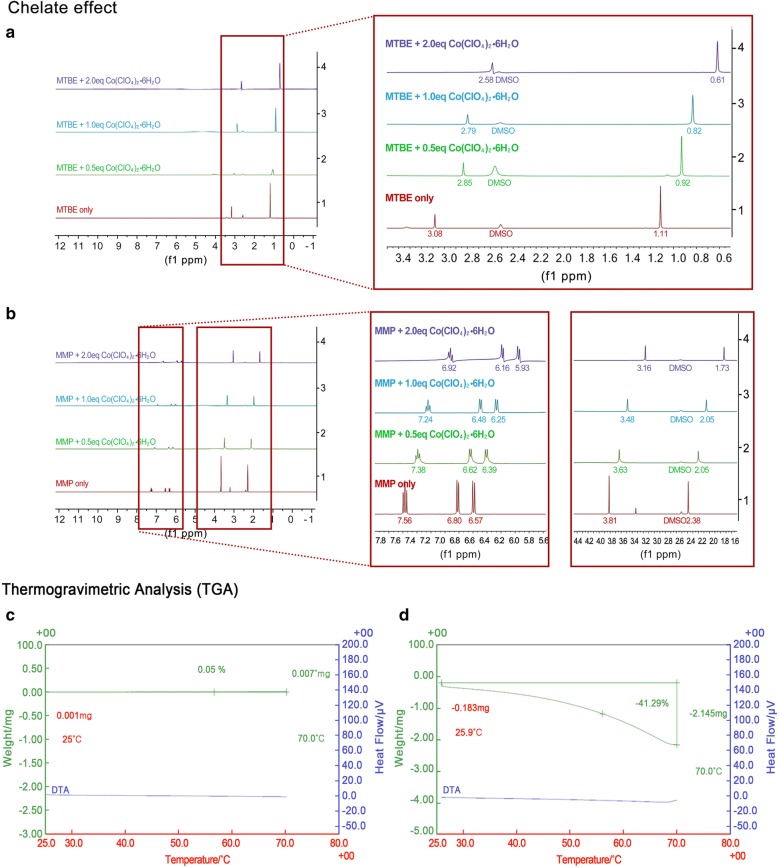
Validation of the chelate effect and thermogravimetric analysis of each solvent. a Determination of intrinsic proton chemical shifts of MTBE in the presence of Co2+ cation using proton nuclear magnetic resonance (NMR). Net chemical shift values (Δδ) of MTBE with increasing Co2+ cation concentrations were 0.19 (0–0.5 eq. Co2+), 0.10 (0.5–1.0 eq. Co2+), and 0.21 (1.0–2.0 eq. Co2+). b Determination of intrinsic proton chemical shifts of MMP in the presence of Co2+ cation using proton nuclear magnetic resonance (NMR). Net chemical shift values (Δδ) of MMP with increasing Co2+ cation concentrations were 0.18 (0–0.5 eq. Co2+), 0.14 (0.5–1.0 eq. Co2+), and 0.32 (1.0–2.0 eq. Co2+). There was no difference in the chemical shifts between MTBE and MMP in the presence of 0.5 equivalent Co2+. However, the chemical shift of MMP was more prominently increased, possibly due to the chelation strength between MMP and Co2+. c Thermogravimetric analysis of MTBE. TGA can be used to evaluate the thermal stability of a compound. A thermogravimetric analyzer continuously measures the mass of a substance (MTBE or MMP) while the temperature is changed over time. In the case of MTBE, TGA analysis was impossible because it quickly vaporized in the chamber due to the low boiling point. d Thermogravimetric analysis of MMP. In the case of MMP, it did not vaporize before applying the initial heat due to its higher boiling point of 156 °C, and slowly vaporized as the temperature increased. MMP, 2-methoxy-6-methylpyridine; MTBE, methyl tert-butyl ether; NMR, nuclear magnetic resonance; TGA, thermogravimetric analysis