Figure 6.
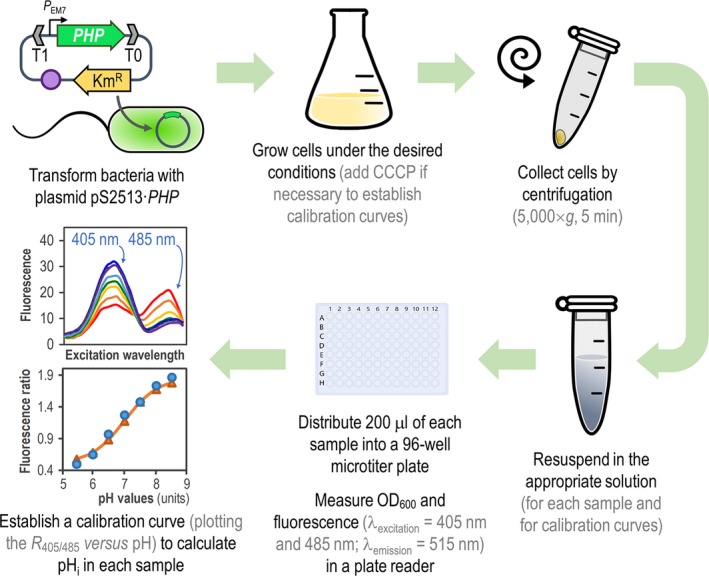
General procedure for in vivo assessment of intracellular pH (pH i) in Pseudomonas species by means of the PHP indicator. Plasmid pS2513·PHP is introduced into the bacteria of interest by chemical transformation, mating or electroporation. The expression of PHP is driven by the constitutive PEM 7 promoter, and vector pSEVA2513 carries the oriV(RSF1010) origin of replication, indicated as a purple circle. The kanamycin‐resistance (KmR) determinant and the T1 and T0 transcriptional terminators are likewise highlighted in the plasmid. Cells are grown in the appropriate culture medium, and a small culture sample is harvested by centrifugation. The cell pellet is resuspended in M9 medium salts to a final optical density at 600 nm (OD 600) = 0.4–0.5. To establish the calibration curve, a second culture is prepared in the same culture medium supplemented with carbonyl cyanide m‐chlorophenyl hydrazine (CCCP). Cells from this culture are also harvested by centrifugation and equilibrated in M9 medium salts adjusted to different pH values (ranging from pH = 5.5–8.5, see Experimental Procedures ) to a final OD 600 = 0.4–0.5. A 200‐μl aliquot of each cell suspension is transferred into a 96‐well microtiter plate, and OD 600 and fluorescence (λexcitation = 405 nm and 485 nm; λemission = 515 nm) are measured in a plate reader. Finally, the R 405/485 values are plotted versus the pH values of pH‐equilibrated cells (calibration curve), and the pH i is calculated using a Boltzmann sigmoid best‐fitting equation.
