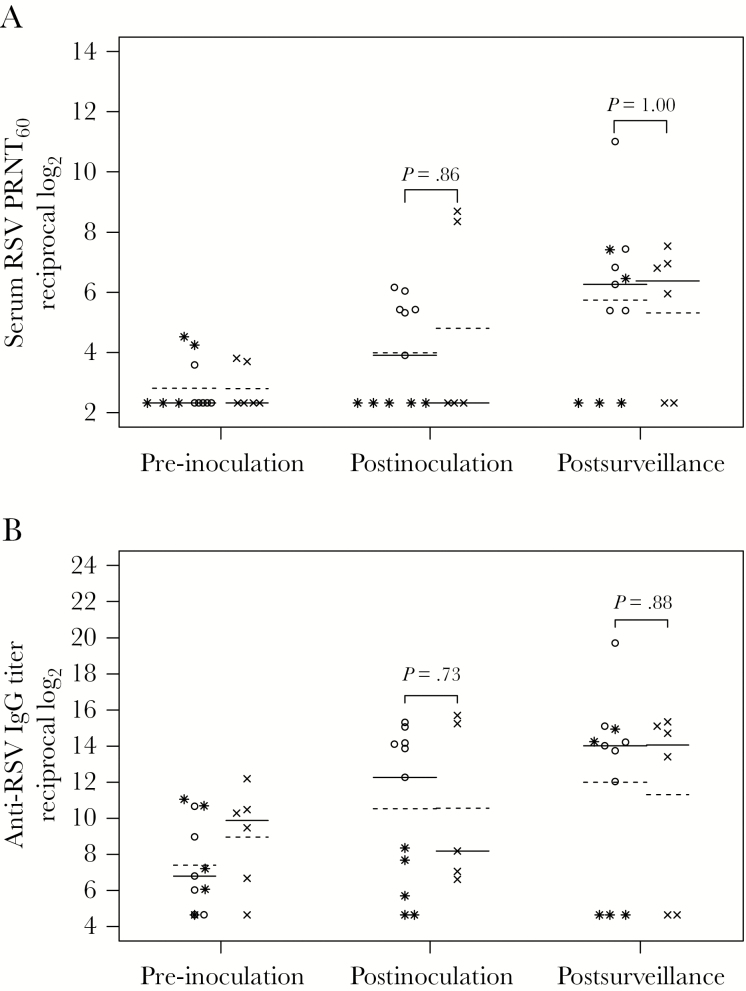
Figure 1.
Serum respiratory syncytial virus (RSV) antibody titers in vaccine and placebo recipients. Serum RSV 60% plaque reduction neutralizing titers (PRNT60) (A) and anti-RSV F IgG titers (B) were determined by complement-enhanced 60% plaque reduction neutralization assay and IgG-specific enzyme-linked immunosorbent assay against purified baculovirus-expressed F protein (provided by Novavax, Inc., Gaithersburg, MD), respectively, for vaccine (open circles and stars) and placebo (x’s) recipients in sera collected at pre-inoculation (screening), postinoculation (study day 56), and postsurveillance (after the RSV season, April 1 to 30 in the calendar year after the inoculation). Titers are expressed as the reciprocal log2. The lines indicate median (solid line) and mean (dashed line) values. P values were determined by Wilcoxon rank-sum test. Five vaccine recipients who did not shed vaccine virus are indicated with the star symbol. The data from the postinoculation visit are missing for 1 placebo recipient.