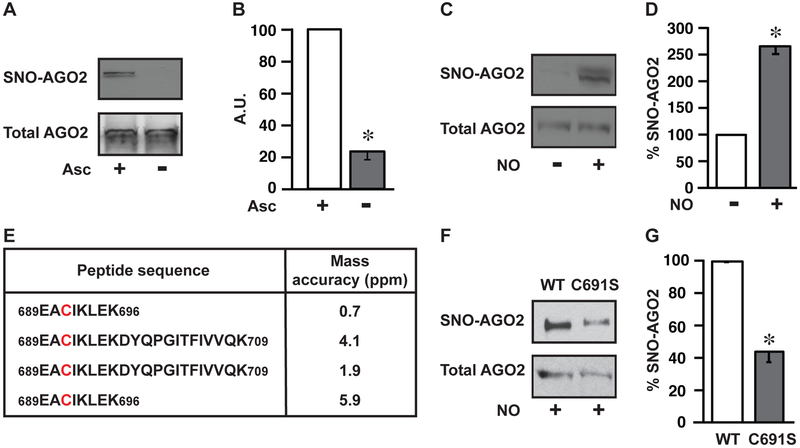
Figure 2. AGO2 Cys691 is a primary locus of S-nitrosylation.
(A) Endogenous S-nitrosylation of human AGO2. Immunoblot for AGO2 in HEK293 cells following SNO-RAC ± ascorbate control (Asc). AGO2 loading control is shown. Gels are representative of three experiments. (B) Quantification of gels in A (n=3, ± SEM). A.U., arbitrary units. *, differs from +Asc by ANOVA with Dunnett’s test (p < 0.05). (C) S-nitrosylation of AGO2 by exogenous NO. Immunoblot for AGO2 in HEK293 cells following SNO-RAC ± NO donor (CysNO). Total AGO2 loading control is shown. Gels are representative of three experiments. (D) Quantification of gels in C (n=3, ± SEM). *, differs from –CysNO by ANOVA with Dunnett’s test (p < 0.05). (E) Locus of S-nitrosylation in AGO2. Peptides containing the Cys691 site of S-nitrosylation identified by LC-MS/MS from 4 independent experiments. SNO-Cys were labelled with iodoacetamide using switch methodology (Jaffrey and Snyder, 2001). Conditions are as in C. (F) Validation of AGO2-Cys691 S-nitrosylation by site-directed mutagenesis. Immunoblot for SNO-AGO2 in HEK293 cells transfected with either WT-AGO2 (WT) or Cys691 mutant AGO2 (C691S) after treatment with CysNO, as in C. Total AGO2 loading control is shown. Gels are representative of three experiments. (G) Quantification of gels in F (n=3, ± SEM). *, differs from WT by ANOVA with Dunnett’s test (p < 0.05). See also Figure S1.