Abstract
New-onset post-transplant diabetes mellitus (PTDM) occurs frequently following allogeneic hematopoietic cell transplant (HCT). Although calcineurin inhibitors and corticosteroids are assumed the cause for hyperglycemia, patients developing PTDM have elevated fasting C-peptide levels before HCT and prior to immunosuppressive medications. To determine if PTDM results from established insulin resistance present before transplant, we performed oral glucose tolerance tests (OGTT) and measured whole-body, peripheral, and hepatic insulin sensitivity with euglycemic hyperinsulinemic clamps before and 90 days after HLA-identical sibling donor HCT in 20 patients without pre-transplant diabetes. HCT recipients were prospectively followed for the development of new-onset PTDM defined as a weekly fasting blood glucose ≥ 126mg/dL or random blood glucose ≥ 200mg/dL. During the first 100 days, all patients received calcineurin inhibitors and 11 (55%) individuals were prospectively diagnosed with new-onset PTDM. PTDM diagnosis preceded corticosteroid treatment. During the pre-transplant OGTT, elevated fasting (87 mg/dl vs. 101 mg/dl; p= 0.005) but not 2-hour post-prandial glucose levels predicted PTDM diagnosis (p= 0.648). In response to insulin infusion during the euglycemic hyperinsulinemic clamp, patients developing PTDM had lower whole-body glucose utilization (p= 0.047) and decreased peripheral/skeletal muscle uptake (p= 0.031) before and after transplant, respectively when compared to non-PTDM patients. Hepatic insulin sensitivity did not differ. Survival was decreased in PTDM patients (2-year estimate: 55% vs. 100%; p=0.039). Insulin resistance before HCT is a risk factor for PTDM independent of immunosuppression. Fasting pre-transplant glucose levels identified PTDM susceptibility and peripheral insulin resistance could be targeted for prevention and treatment of PTDM after HCT.
Keywords: Post-transplant Diabetes Mellitus, Allogeneic Hematopoietic Cell Transplant, Insulin Resistance
Introduction:
Allogeneic hematopoietic cell transplantation (HCT) is an effective therapy for patients with high risk hematological malignancies, however its efficacy can be diminished by treatment related complications. The mortality risk increases 3-fold for the 50% of patients developing new-onset post-transplant diabetes mellitus (PTDM) after HCT (1, 2). Despite the clinical burden of this disease, PTDM physiology remains poorly understood and recommendations for screening are negligible. Current dogma suggests that PTDM is merely a side effect of diabetogenic immunosuppressive medications. Contrary to this belief, we have shown that elevated fasting C-peptide levels prior to transplant (i.e., before immunosuppression or alloreactivity) predicts both PTDM and mortality (1). Although our C-peptide data indirectly supports the role of established insulin resistance in PTDM development, independent of immunosuppression, formal techniques are available to directly measure insulin sensitivity in vivo, including the oral glucose tolerance test (OGTT) and the euglycemic hyperinsulinemic clamp.
Insulin resistance and β-cell failure are the major determinants of diabetes progression. Insulin resistance is categorized broadly as hepatic or peripheral (3). In the setting of insulin resistance, hyperglycemia results from insulin’s failure to adequately suppress hepatic glucose production and/or increase glucose uptake by skeletal muscle. During a euglycemic hyperinsulinemic clamp, patients receive a fixed dose of intravenous insulin continuously while plasma glucose is held constant at 90–100 mg/dl (defined as euglycemia) using a variable infusion of 20% dextrose. Insulin’s action in vivo can be calculated by quantifying the amount of dextrose infused to maintain steady-state euglycemia. The greater the insulin sensitivity, the more dextrose required to maintain euglycemia due to insulin’s regulatory effects on liver glucose production and glucose disposal by skeletal muscle. The euglycemic hyperinsulinemic clamp is considered the gold standard for determining insulin sensitivity and when combined with a radiolabeled glucose tracer infusion, it can quantify both hepatic and peripheral/skeletal muscle insulin resistance (4–6). In this study, we performed a hyperinsulinemic clamp and an OGTT on HCT recipients to determine if 1) new-onset PTDM develops from established insulin resistance and 2) whether plasma glucose levels during a standard oral glucose challenge can predict PTDM diagnosis. These results could be used to identify high risk patients prior to HCT or find therapeutic targets for the prevention of new-onset PTDM.
Methods:
Patients and study protocol.
From November 2014 to February 2017, 79 HCT candidates were screened, 35 individuals provided consent, and 15 were excluded (Figure 1). In total, 20 euglycemic patients (≥18 years old) with hematologic malignancies undergoing myeloablative or reduced intensity conditioning followed by a related, HLA-identical, unmanipulated peripheral blood HCT were accrued into an IRB-approved study. No patients received thymoglobulin or total body irradiation (TBI). Patients with a history of diabetes mellitus, diabetes therapy within 6 months of enrollment, fasting blood glucose ≥ 126 mg/dL at screening, a 2-hour post-prandial blood glucose ≥ 200 mg/dL during the pre-transplant OGTT, chronic/continuous corticosteroid treatment at evaluation (>10 mg/day of prednisone or prednisone equivalent) were excluded. Patients previously treated with intermittent/pulsed corticosteroids were permitted. Baseline OGTT and hyperinsulinemic clamps were obtained prior to the start of conditioning/chemotherapy/immunosuppressive medications and were repeated 90 days after HCT. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus and either methotrexate or mycophenolate mofetil. Acute and chronic GVHD were assessed using consensus criteria (7, 8). From day 0 to day+100, fasting blood glucose levels were monitored weekly and random blood glucose levels were assessed daily until neutrophil engraftment and then at least 2–3 times a week thereafter. During the first 100 days of transplant, HCT recipients were monitored for GVHD, corticosteroid treatment for any reason, and new-onset PTDM defined as a weekly fasting blood glucose ≥ 126 mg/dL, any random blood glucose ≥ 200 mg/dL, or a 2-hour post-prandial glucose ≥ 200 mg/dL during an OGTT (1, 9). All HCT recipients were monitored prospectively for PTDM, however 1 patient did not undergo a hyperinsulinemic clamp due to lack of peripheral intravenous access, and 2 patients were unable to complete the second hyperinsulinemic clamp due to logistical considerations and malignancy relapse after HCT.
Figure 1.
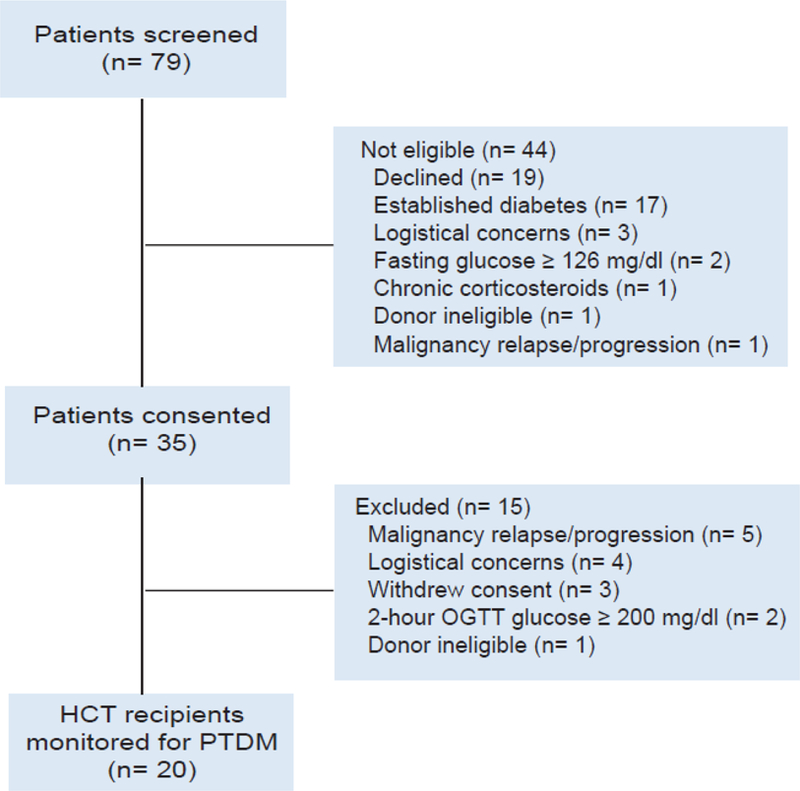
Screening and disposition of possible study patients who were evaluated for related, HLA-identical, peripheral blood hematopoietic cell transplant (HCT). Logistical considerations included: moving patient to Nashville, Tennessee, scheduling pre-transplant visits at Vanderbilt Clinical Research Center, central venous catheter placement, coordinating care with donor apheresis team and stem cell processing lab. OGTT, oral glucose tolerance test; PTDM, post-transplant diabetes mellitus.
Study procedures.
HCT patients completed OGTT and hyperinsulinemic clamp at the Vanderbilt Clinical Research Center on separate days before starting chemotherapy/immunosuppression and not more than 14 days before transplant. These baseline studies measured insulin sensitivity without confounding by alloreactivity, immunosuppression, or other features of the transplant. The procedures were repeated 90 days post-HCT. Additional information on the conduct of OGTTs/hyperinsulinemic clamps, calculations of insulin sensitivity, and statistical analysis are available in the supplemental methods.
Study approval.
The protocol was approved by the Vanderbilt University Medical Center Institutional Review Board. All subjects gave written informed consent and were treated in accordance with the Declaration of Helsinki.
Results:
OGTTs and euglycemic hyperinsulinemic clamps were performed before chemotherapy conditioning and 90 days after matched related donor HCT in 20 patients without pre-existing diabetes. After transplant, HCT recipients were monitored prospectively for PTDM, defined as a weekly fasting blood glucose ≥ 126 mg/dL, any random blood glucose ≥ 200 mg/dL, or a 2-hour post-prandial OGTT glucose ≥ 200 mg/dL. Within the first 100 days of transplant, new-onset PTDM was diagnosed in 11 (55%) patients at a median of 22 days post-HCT (range, 0–88 days). Table 1 describes clinical characteristics. Neither transplant for a lymphoid malignancy nor previous treatment with corticosteroids increased PTDM risk (Table 1). All PTDM developed before grade 2–4 acute GVHD except for 2 (18%) cases, and no patients were receiving corticosteroids when diagnosed with diabetes. Neither the diagnosis of acute/chronic GVHD nor treatment with corticosteroids was associated with PTDM (Table 1). Parenteral nutrition was not used.
Table 1.
Characteristics of patients undergoing related, HLA-identical, peripheral blood hematopoietic cell transplant, stratified for PTDM development
| Variable | No PTDM n= 9 (%) | PTDM n= 11 (%) | P-value |
|---|---|---|---|
| Median age, years (range) | 56 (30–61) | 55 (26–66) | 0.790 |
| Male | 6 (67) | 9 (82) | 0.617 |
| Caucasian ethnicity | 7 (78) | 10 (91) | 0.566 |
| Diabetes family history | 1 (11) | 4 (36) | 0.319 |
| Median pre-HCT BMI, kg/m2 (range) | 27.2 (22.4–48.9) | 30.3 (22.5–36.9) | 0.210 |
| Median day+90 BMI, kg/m2 (range) | 24.4 (19.8–46.3) | 28.7 (20.1–32.9) | 0.201 |
| Malignancy | |||
| Myeloid | 5 (56) | 7 (64) | 1.00 |
| AML/MDS (n= 8) | |||
| CML/MPN (n= 4) | |||
| Lymphoid | 4 (44) | 4 (36) | |
| NHL (n= 7) | |||
| CML lymphoid blast crisis (n= 1) | |||
| Pre-HCT steroid treatment | 3 (33) | 5 (45) | 0.67 |
| Chemotherapy | |||
| Myeloablative | 4 (44) | 4 (36) | 1.00 |
| Reduced intensity | 5 (56) | 7 (64) | |
| GVHD Prophylaxis | |||
| FK+MTX | 5 (56) | 7 (64) | 1.00 |
| FK+MMF | 4 (44) | 4 (36) | |
| Grade 2–4 acute GVHD | 4 (44)A | 5 (45)A | 1.00 |
| Steroid treatment first 100 days | 5 (56)B | 3 (27)B | 0.370 |
| Max steroid dose, mg/kg (range) | 0.4 (0.1–0.5) | 0.5 (0.1–1) | 0.539 |
| NIH mod-severe chronic GVHD | 5 (56) | 7 (64) | 1.00 |
Grade 2–4 GVHD treatment: systemic corticosteroids (prednisone or methylprednisolone) (n= 5), beclomethasone diproprionate and budesonide (n= 4).
Indications for systemic corticosteroid (prednisone or methylprednisolone) treatment: grade 1–4 GVHD (n= 6), pneumonitis/pleurisy (n= 1), and gout (n= 1).
PTDM, post-transplant diabetes mellitus; HCT, hematopoietic cell transplant; BMI, body mass index; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; MPN, myeloproliferative neoplasm; NHL, non-hodgkin’s lymphoma; FK, tacrolimus; MTX, methotrexate; MMF, mycophenolate mofetil; GVHD, graft-versus-host disease. NIH, National Institutes of Health; Mod, moderate.
During the baseline OGTT, impaired fasting glucose (fasting plasma glucose of 100–125 mg/dl) and impaired glucose tolerance (2-hour plasma glucose concentration of 140–199 mg/dl) after a 75 g glucose load was observed in 5 (25%) and 7 (35%) of patients, respectively. When analyzed as either a continuous or dichotomous variable (normal vs. impaired glucose tolerance), the pre-transplant 2-hour OGTT plasma glucose values did not discriminate between patients developing PTDM and those remaining non-diabetic after HCT. However, pre-transplant fasting glucose and C-peptide levels were elevated in patients diagnosed with PTDM (Table 2). Furthermore, all 5 patients with impaired fasting glucose compared to only 6 out of 15 individuals with normal fasting glucose levels progressed to PTDM after HCT (p= 0.038). During the second OGTT after HCT, diabetic, impaired, and normal fasting or 2-hour postprandial glucose levels were observed in 7 vs. 0, 3 vs. 7, and 1 vs. 2 of the PTDM and non-PTDM patients, respectively (Table 2).
Table 2.
Oral glucose tolerance test results from recipients of related, HLA-identical, peripheral blood hematopoietic cell transplant, stratified for PTDM development
| Variable | No PTDM n= 9 | PTDM n= 11 | P-value |
|---|---|---|---|
| Fasting glucose (mg/dl) | |||
| Pre-HCT | 87 + 2.61 | 101 + 3.00 | 0.005 |
| Post-HCT | 103 + 6.07 | 118 + 6.98A | 0.204 |
| 2-hour post-prandial glucose (mg/dl) | |||
| Pre-HCT | 124 + 9.78 | 129 + 9.23 | 0.648 |
| Post-HCT | 151 + 10.6 | 211 + 18.8A | 0.008 |
| Fasting C-peptide (ng/ml) | |||
| Pre-HCT | 2.03 + 0.34 | 2.97 + 0.40 | 0.074 |
| Post-HCT | 3.04 + 0.54 | 4.28 + 0.52A | 0.039 |
| 2-hour post-prandial C-peptide (ng/ml) | |||
| Pre-HCT | 6.30 + 0.97 | 7.48 + 0.83 | 0.210 |
| Post-HCT | 6.75 + 1.15 | 11.3 + 2.14A | 0.052 |
Data expressed as mean + SEM.
After transplant, fasting glucose (P= 0.014), 2-hour post-prandial glucose (P= 0.003), fasting C-peptide level (P= 0.003), and 2-hour post-prandial C-peptide level (P=0.016) were increased in PTDM patients when compared to their respective baseline results. Otherwise before and after transplant values did not differ for within group comparisons.
PTDM, post-transplant diabetes mellitus; HCT, hematopoietic cell transplant.
To directly measure glucose utilization and insulin sensitivity, hyperinsulinemic clamps were performed. During insulin stimulation, HCT recipients who developed PTDM exhibited lower whole-body glucose utilization (M) before transplant and decreased muscle glucose uptake (Rd) after transplant when compared to individuals never diagnosed with PTDM (Figure 2). During the high dose insulin phase, whole-body insulin sensitivity and peripheral/skeletal muscle insulin sensitivity was calculated as the exogenous glucose infusion rate per unit of plasma insulin (M/I) or as the rate of isotopically determined disappearance of glucose per unit of plasma insulin (Rd/I), respectively. Patients developing new-onset PTDM exhibited decreased peripheral/skeletal muscle and whole-body insulin sensitivity after transplant (Figure 3). Differences were not detected in hepatic glucose production (HGP) or hepatic insulin sensitivity index (HISI) before or after HCT among patients with or without PTDM (Supplemental table 1). Since baseline measurements of insulin sensitivity could influence post-HCT comparisons, ordinary regression was performed. After adjustment for baseline/pre-transplant values, the regression analyses confirmed that PTDM patients had both lower whole-body (β= −28.0; 95% CI, −47.2 to −8.9; p= 0.013) and peripheral/skeletal muscle insulin sensitivity (β= −58.1; 95% CI, - 97.0 to −19.3; p= 0.012) during the high dose insulin infusion after HCT.
Figure 2.
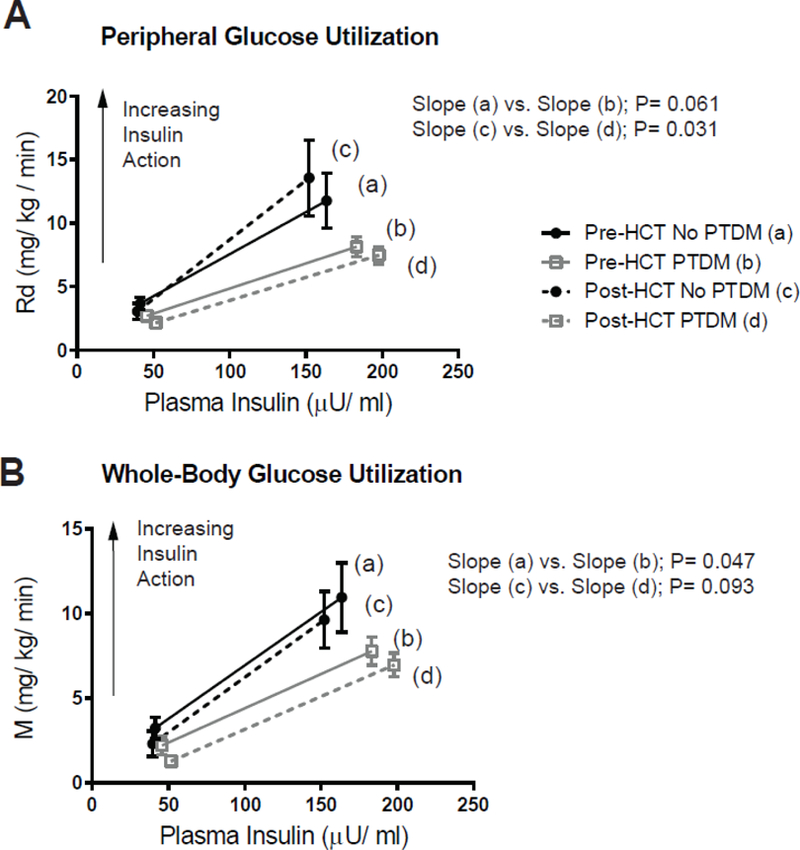
Insulin-stimulated glucose utilization before and 90 days after related, HLA-identical, peripheral blood hematopoietic cell transplant (HCT). Peripheral/skeletal muscle (Rd) and whole-body glucose utilization (M) were calculated during the euglycemic insulin clamp studies and plotted against the plasma insulin level (A and B, respectively). Data expressed as mean ± SEM for n= 8 for pre-HCT measurements and n= 6 for post-HCT measurements in the “No PTDM” group and n= 11 for the “PTDM” cohort. Data were stratified for the development of new-onset post-transplant diabetes mellitus (PTDM). The slopes of the lines were compared using generalized least squares model.
Figure 3.
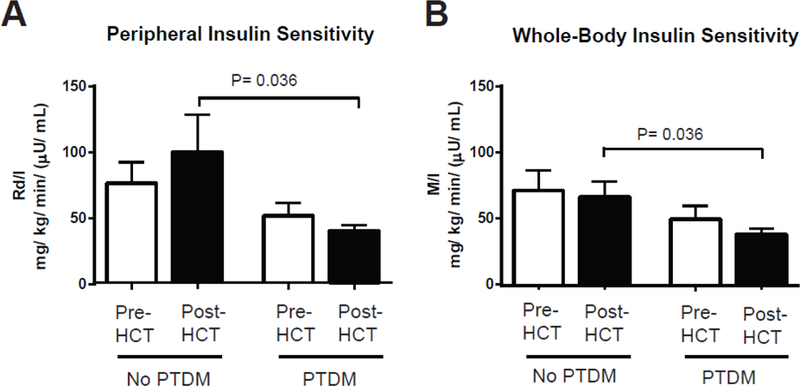
Insulin sensitivity before and 90 days after related, HLA-identical, peripheral blood hematopoietic cell transplant (HCT). Peripheral/skeletal muscle (Rd/I) and whole-body insulin sensitivity (M/I) were calculated during the euglycemic insulin clamp studies with high dose insulin infusion (80 mU/m2/min) (A and B, respectively). Decreased Rd/I and M/I values reflect worsening insulin resistance and decreased glucose disposal. Data expressed as mean ± SEM for n= 8 for pre-HCT measurements and n= 6 for post-HCT measurements in the “No PTDM” group and n= 11 for the “PTDM” cohort. Data were stratified for the development of new-onset post-transplant diabetes mellitus (PTDM). Independent and dependent groups were compared with a Mann-Whitney U test and Wilcoxon signed rank test, respectively.
To investigate the previously noted association between PTDM and inferior HCT outcomes, an exploratory landmark survival analysis was performed (1, 2). The median follow-up for surviving patients was 21 months. Survival curves were created beginning at day+100 after both PTDM assessment and second OGTTs were complete. Estimated 2-year OS was decreased in patients developing PTDM compared with non-PTDM individuals 55% (95% CI, 23%−83%) vs 100% (95% CI, 63%−100%) (p= 0.039) and in patients with day+90 fasting hyperglycemia 0% (95% CI, 0%−71%) vs. 82% (95% CI, 48%−98%) vs. 100% (95% CI, 48%−100%) (p< 0.001) representing survival for individuals with either diabetic, impaired, or normal fasting glucose levels, respectively (Figure 4).
Figure 4.
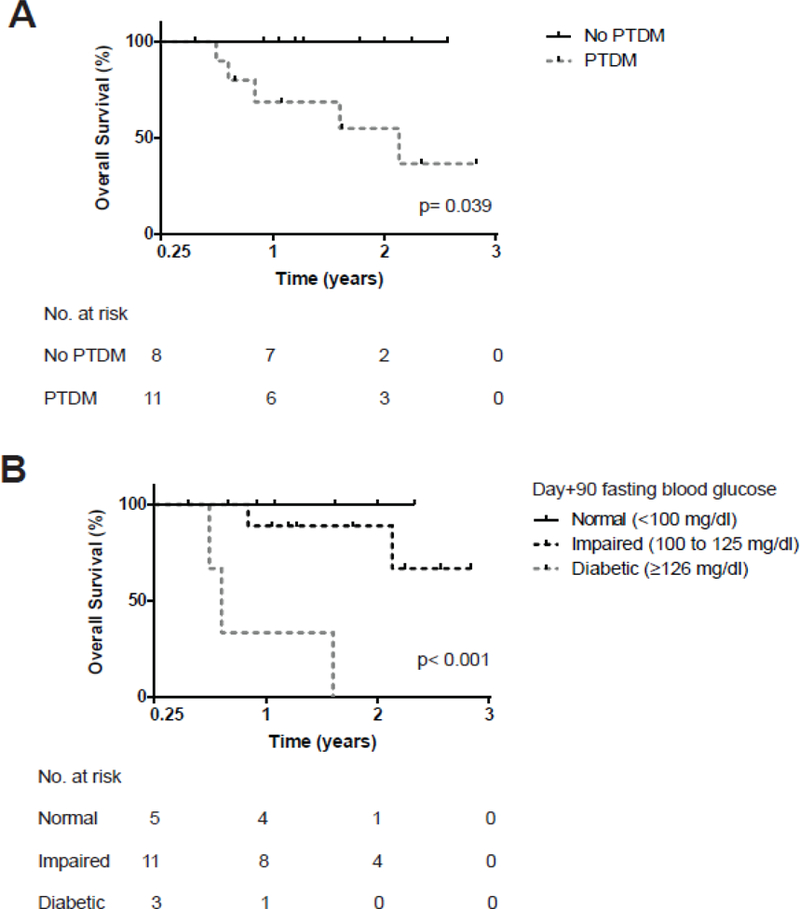
Kaplan-Meier estimates of overall survival beginning 100 days after related, HLA-identical, peripheral blood hematopoietic cell transplant (HCT). Survival curves were stratified for the development of new-onset post-transplant diabetes mellitus (PTDM) during the first 100 days of transplant (A) or by fasting blood glucose level during the day+90 oral glucose tolerance test (B). Groups were compared with a log-rank test. Causes of death included: graft-versus-host disease (n= 2), malignancy relapse (n= 1), infection (n= 1), and myocardial infarction (n= 1).
Discussion:
The focus of the current study was to use OGTTs and euglycemic hyperinsulinemic clamps to define the onset and mechanisms causing glucose dysregulation during HCT. Although, clinical risk factors for new-onset PTDM including: older age, non-white ethnicity, corticosteroids, parenteral nutrition, and TBI have been previously discussed by our group and others, the actual mechanisms causing PTDM remain unknown (1, 10–12). Our OGTT and clamp results reveal that risk of progression to PTDM after HCT depends on the recipient’s insulin resistance status before transplant. Specifically, PTDM pathophysiology is characterized by subtle whole-body insulin resistance before transplant followed by more robust evidence of peripheral/skeletal muscle insulin resistance after HCT. These results are remarkable for several reasons. By demonstrating pre-HCT insulin resistance as a determinant for PTDM development, we refute the misperception that PTDM is merely a side effect of immunosuppressive medications. The study identifies skeletal muscle insulin resistance as a target for therapeutic intervention to prevent glucose dysregulation and to limit transplant morbidity. Not only is a target identified, but we also show that generalized insulin resistance manifesting as fasting hyperglycemia could be used to predict PTDM.
A limitation of this research is the sample size. In general clamp studies are smaller but the results from this technique remain very reproducible (13). Although the number of study participants was small, the current research is further supported by our previous prospective study in a cohort of 84 HCT patients which showed nearly identical results with PTDM incidence of 60% vs. 55%, median time to PTDM diagnosis of 23 days vs. 22 days, and 2-year survival of PTDM patients of 54% vs. 55% for the past and present study, respectively (1). The high incidence and early diagnosis of PTDM especially before corticosteroid initiation likely reflects vigilant surveillance and strict diagnostic criteria, however multicenter trials will be needed for confirmation.
Other common concerns for clinical research include treatment heterogeneity and generalizability of results. Our current study focused on a uniform group of human leukocyte antigen (HLA)-identical sibling donor transplants receiving non-TBI conditioning and tacrolimusbased immunosuppression. All patients undergoing HCT were screened for clinical trial eligibility. However our cohort was composed predominantly of male Caucasians, which may reflect biases inherent to HCT or may represent the demographics of our clinical practice. The effects of this population bias is unknown, however gender was not previously associated with PTDM (1). Given the similarities in PTDM incidence between the current study with our past research which included a more heterogeneous cohort, we suspect that our clamp data can be extrapolated to other types of transplants. Furthermore the study was designed so that differences in HCT techniques (i.e. conditioning intensity) or alloreactivity/immunosuppression could not affect the OGTT and clamp results obtained before transplant.
The HCT-comorbidity index, a commonly used clinical tool has identified diabetes treatment before transplant as a predictor of decreased survival after HCT (14). Similarly our current and previous research has shown a relationship between new-onset PTDM and transplant mortality (1, 2). In general, diabetes is an accepted risk factor for cardiovascular, kidney, and infectious diseases, however the physiology of hyperglycemia is complex. Insulin resistance is characterized by low grade inflammation which could have negative consequences during HCT (15, 16). Diabetes may also influence malignancy relapse or the development of secondary cancers after transplant via epigenetic pathways. Recent studies have shown that hyperglycemia prevents AMP-activated kinase (AMPK) phosphorylation and stabilization of the tumor suppressor TET2 leading to cancer susceptibility, an abnormality which is reversed by metformin (17). Our preliminary survival data is consistent with past results and confirms other research demonstrating inferior survival among HCT recipients with hyperglycemia (1, 2, 18, 19). Larger studies will be needed to elucidate the cause-specific mortality for HCT recipients developing new-onset PTDM.
Although we demonstrated evolving insulin resistance in the development of PTDM the exact etiology for impaired insulin sensitivity during HCT remains unclear. Pre-transplant treatment could contribute to impaired insulin sensitivity either directly via corticosteroid exposure or indirectly by inducing detrimental changes in diet and exercise Both increase in fat mass or loss of muscle mass with initial therapy or during transplant could lead to reduced glucose utilization. Accordingly, BMI tended to be higher in the PTDM group (Table 1) and despite weight loss during transplant, there was no improvement in insulin sensitivity (Figure 2). Alternatively, preclinical studies indicate that low-grade inflammation is a driver of insulin resistance in nontransplant animal models (15, 16). Analogously, PTDM also may result from underlying immune dysregulation that worsens after transplant (2, 20). The OGTT and clamp data demonstrate changes in glucose metabolism before transplant, indicating that PTDM is distinct from alloreactivity or its treatment with corticosteroids. It remains possible that immunosuppressive medications/corticosteroids could “unmask” subtle, established metabolic changes that are otherwise discernable only with fasting labs or insulin clamps. Given the limitations of the current investigation as well as the complexities of PTDM, animal models and human studies pairing clamps with muscle biopsies likely will be needed to define the molecular cause and specific role of skeletal muscle insulin resistance in PTDM development.
This study enhances our understanding of PTDM, a disease that affects about half of HCT recipients and increases the mortality risk 3-fold (1, 2). The pre-transplant OGTT and clamp data refutes the commonly held misperception that PTDM is simply a side effect of immunosuppressive medications. Our results demonstrate that elevated fasting pre-transplant glucose levels may identify PTDM risk and that pharmacological therapies or lifestyle interventions targeting skeletal muscle insulin resistance should be studied for the prevention and treatment of new-onset PTDM after HCT. For example, medical fitness facilities specializing in patients with chronic health conditions (i.e. cardiac rehabilitation) could be leveraged for peritransplant exercise training. Even short-term exercise for 7 days can improve insulin sensitivity (6). The Diabetes Prevention Program was a randomized clinical trial comparing lifestyle intervention [7% weight reduction and moderate physical exercise (i.e. brisk walk) for 150 minutes per week] or metformin 850 mg twice per day with placebo in a high-risk non-diabetic population with elevated fasting and post-prandial plasma glucose concentrations. After 15 years of follow up, diabetes incidence was reduced 18–27% with metformin or lifestyle interventions when compared to placebo (21). These studies lay the ground work for a prospective trial selecting or stratifying HCT participants based on impaired fasting glucose, then investigating insulin sensitizers such as biguanides or thiazolidinediones initiated pre-transplant on PTDM development and transplant mortality.
Supplementary Material
Highlights.
Insulin resistance before HCT is a risk factor for PTDM independent of immunosuppression Fasting pre-transplant glucose levels identified PTDM susceptibility
Insulin resistance can be targeted for prevention and treatment of PTDM after HCT
Acknowledgements
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Grant 1K23HL122143-01A (B.G.E.), National Institutes of Health: UL1TR000445 (Vanderbilt CTSA Award) and from National Institute of Diabetes and Digestive and Kidney Diseases DK020593 and DK059637 (Vanderbilt Diabetes Research and Training Center).
The authors would like to thank the patients who volunteered for this study, their families, the nurses, and the nurse practitioners who cared for them. We would also like to thank Kareem Jabbour, BS, Joseph Antoun, PhD, and Pam Marks-Shulman MS, RD for their technical and intellectual assistance with the hyperinsulinemic clamps.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.Griffith ML, Jagasia MH, Misfeldt AA, Chen H, Engelhardt BG, Kassim A, et al. Pretransplantation C-Peptide level predicts early posttransplantation diabetes mellitus and has an impact on survival after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2011;17(1):86–92. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt BG, Jagasia SM, Crowe JE Jr., Griffith ML, Savani BN, Kassim AA, et al. Predicting posttransplantation diabetes mellitus by regulatory T-cell phenotype: implications for metabolic intervention to modulate alloreactivity. Blood 2012;119(10):2417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37(6):667–87. [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 5.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 1956;187(1):15–24. [DOI] [PubMed] [Google Scholar]
- 6.Winnick JJ, Sherman WM, Habash DL, Stout MB, Failla ML, Belury MA, et al. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J Clin Endocrinol Metab 2008;93(3):771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8. [PubMed] [Google Scholar]
- 8.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21(3):389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diagnosis and classification of diabetes mellitus. Diabetes Care 33 Suppl 1:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood 2007;109(4):1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009;15(9):1100–7. [DOI] [PubMed] [Google Scholar]
- 12.Sheean PM, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006;12(6):656–64. [DOI] [PubMed] [Google Scholar]
- 13.Heise T, Zijlstra E, Nosek L, Heckermann S, Plum-Morschel L, Forst T. Euglycaemic glucose clamp: what it can and cannot do, and how to do it. Diabetes Obes Metab 2016;18(10):962–72. [DOI] [PubMed] [Google Scholar]
- 14.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15(8):921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15(8):930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 2018;559(7715):637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation 2007;84(7):814–20. [DOI] [PubMed] [Google Scholar]
- 19.Hammer MJ, Casper C, Gooley TA, O’Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant 2009;15(3):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnpulle RA, Paczesny S, Jung DK, Daguindau E, Jagasia MH, Savani BN, et al. Metabolic Complications Precede Alloreactivity and Are Characterized by Changes in Suppression of Tumorigenicity 2 Signaling. Biol Blood Marrow Transplant 2017;23(3):529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Prevention Program Research G. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3(11):866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


