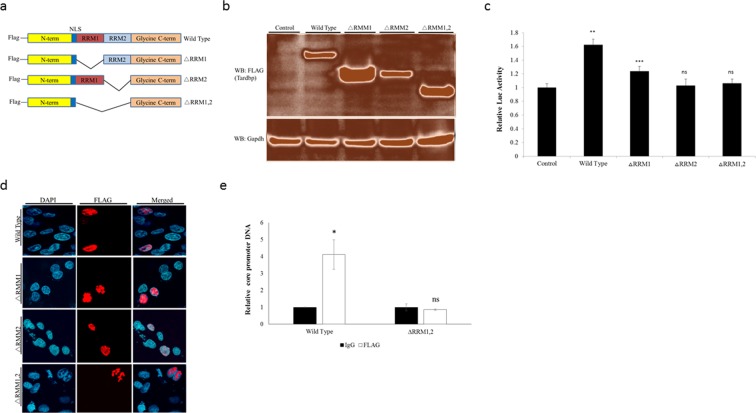
Figure 5.
The RNA recognition motifs (RRMs) of TARDBP are crucial for activation of the core promoter. (a) A schematic structure of the wild type (WT) TARDBP showing the location of the FLAG tag, the two RRMs (RRM1 and RRM2) and the nuclear localization signal (NLS). Alongside are the three mutants with deletion of RRM1 (ΔRRM1), RRM2 (ΔRRM1) or both RRM1 and 2 (ΔRRM1, 2). (b) Each of the four plasmids or the control empty vector was transfected into NTCP-HepG2 cells, and after 48 hours protein expression was determined by western blotting using an anti-FLAG antibody. (c) To compare the effect of these proteins on the core promoter activity, the pGL3-CP plasmid was transfected alongside equal amounts of the control plasmid, WT TARDBP, or each of the three RRM mutant plasmids into NTCP-HepG2 cells. Luciferase activities were measured at 48 hours after transfections. Relative values were calculated as described in Fig. 3e. (d) For intracellular localization of the proteins, the plasmids were transfected as in (b) above. After 48 hours, the cells were treated with the anti-FLAG antibody and visualized by immunofluorescence microscopy. The nucleus was visualized with DAPI. (e) To confirm that the two RRMs play a role in interaction of TARDBP with the core promoter, the FLAG tagged WT and the ΔRRM1, 2 mutant proteins were expressed in T23 cells, followed by a ChIP assay as in Fig. 3b using control and anti-FLAG antibodies. The resulting DNA was analyzed with the core promoter primers by qPCR. The relative values were normalized to the amount of DNA precipitated by the control which, was set at one. Results are reported as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 and ns-non significant.